Introduction: Spetzler-Martin grade III arteriovenous malformation (AVM) located in the peri-Rolandic area involves the motor or sensory cortex. Its management is particularly controversial and requires a multimodal approach.
Methods: From September 2018 to March 2022, we reviewed patients harboring grade III AVM located in peri-Rolandic areas that had been treated with microsurgery at the Baghdad Neurosurgery Teaching Hospital. Eighteen parameters were applied to each case, which were categorized into pre-, intra-, and postoperative factors.
Results: A total of 8 Patients are included in this study. The size of the AVM was between 35 mm and 54 mm. Sensory-located AVM was found in 5 cases, and the remaining 3 cases have motor-located AVMs. Total removal of AVM was achieved in 7 cases. 6 patients developed new neurological deficits after surgeries which were resolved in subsequent weeks and the mRS score at follow-up was 1 in all patients.
Conclusion: Microsurgical management of peri-Rolandic Spetzler-Martin grade 3 AVM is challenging; however, it is still more promising in achieving full excision of the lesion.
Grade III arteriovenous malformation, Spetzler-Martin grading system, Peri-Rolandic area, Eloquence, Microsurgery, Radiosurgery, Embolization
AVM: Arteriovenous Malformation; CT: Computed Tomography; GCS: Glasgow Coma Scale; mRS: modified Rank in scale
In 1986, Spetzler and Martin published what has become the predominant classification scheme for brain AVMs [1]. This scheme classifies the AVM into five grades to provide a good stratification regarding morbidity and mortality following the management of AVMs. Of all grades, grade III AVM with eloquence involvement is still a dilemma, and its management is particularly controversial and requires a multimodality approach [2,3]. Many authors support radiosurgical management since it is more appealing and essentially devoid of immediate treatment-related complications [4], while others go with endovascular management, which expands the ability to treat a wider range of AVMs [5].
The microsurgical management of grade III AVMs has been declining in the last few decades, especially after the introduction of minimally invasive procedures. In special cases, where the lesion involves peri-Rolandic areas, Safe microsurgical resection can be challenging, however, promising [6]. In our study, we describe our experience with eight cases harboring an AVM in the peri-Rolandic area which underwent microsurgical resection. We believe there still exists a subgroup of patients with grade III AVMs near the eloquent peri-Rolandic cortex in which this approach may be safe and encouraging.
Our study reviewed patients’ data from September 2018 to March 2022 treated at the Baghdad Neurosurgery Teaching Hospital in Baghdad, Iraq. Patients with Spetzler-Martin grade III AVM located at peri-Rolandic areas that had been treated with microsurgery were included in this study. The peri-Rolandic territory involves the pre- and post-Rolandic areas, which represent the motor and sensory cortex, respectively.
An Analysis of 18 parameters was applied to each case; they are categorized into pre-, intra-, and post-operative factors. The pre-operative parameters include age, gender, presentation, Glasgow Coma Scale (GCS), neurological deficit, and AVM characteristics like location, size, eloquence, and venous drainage. Additionally, intra-operative parameters are also applied, including operation duration, blood loss, and brain swelling. Finally, Postoperative parameters are GCS, neurological deficits, AVM remnant, development of seizures, follow-up period, and modified Rankin Scale (mRS) at follow-up.
From September 2018 to March 2022, a total of 8 Patients harboring a Spetzler-Martin grade III AVM with involvement of peri-Rolandic areas and treated by microsurgery are included in this study.
The patient population included 3 (37.5%) males and 5 (62.5%) females. The range of ages was between 13 and 53 (mean 30.8 years) and 3 patients were in the pediatric age group (≤ 18). The presenting symptom was a seizure in all the patients and no neurological deficit had been encountered preoperatively. All the AVMs had a negative rupture status at the time of presentation (Table 1).
Table 1: Grade III peri-Rolandic AVM; Pre-operative parameters. View Table 1
The size of the AVM was between 35 mm and 54 mm and the venous drainage was superficial in all cases. Sensory-located AVM was found in 5 cases, and the remaining 3 cases have motor-located AVMs. All patients underwent elective surgery and the operation duration was between 7-9 hours. Regarding postoperative parameter, 6 patients developed new neurological deficits (4 weakness and 2 dysphasia), which were resolved in the subsequent follow-up. The follow-up period was 2-3 years and the mRS score at follow-up was 1 in all cases. Only one case has AVM remnant and 2 cases developed a seizure in the follow-up period (Table 2).
Table 2: Grade III peri-Rolandic AVM; Intra and post-operative parameters. View Table 2
We present the first two cases as case scenarios to represent an example of our case series: one is motor AVM (Figure 1) and the other is sensory AVM (Figure 2).
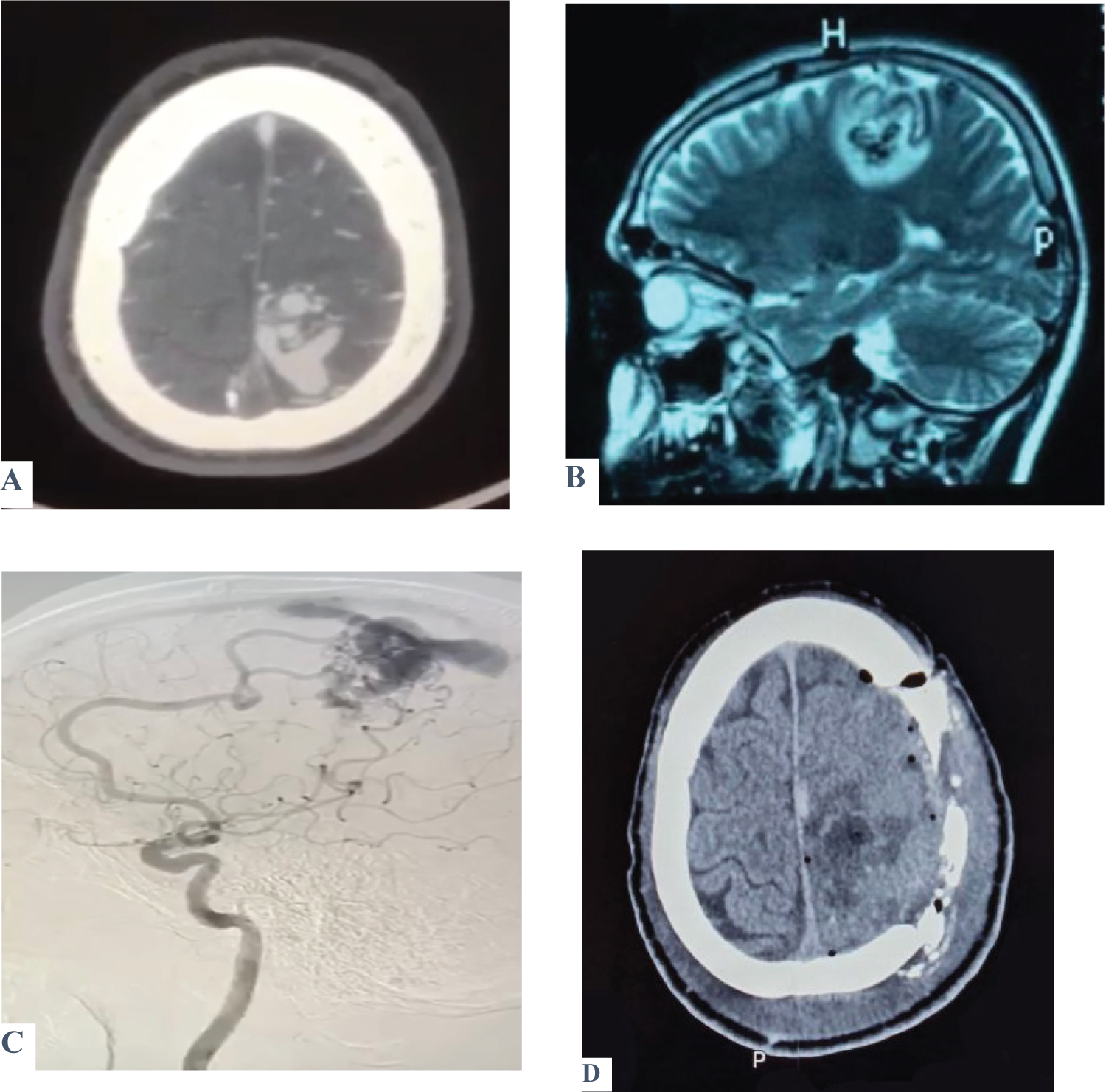 Figure 1: Shows a case of a 30-year-old male presented with two attacks of seizures associated with recurrent episodes of nausea and vomiting. On examination, there was no motor or sensory deficit, Glasgow coma score (GCS) was 15 with no fit or vomiting. A brain CT scan (A) was ordered and showed a left parietal AVM confirmed by MRA (B). DSA was performed to reveal exactly the location and characteristics of the feeding arteries and draining veins (C). Post-operatively, the patient’s GCS was 15 with no fit and only one episode of vomiting. Motor examination revealed a decrease in the initiation of movement; otherwise, the patient was improved. Post-operative CT was done and showed total excision of the AVM (D).
View Figure 1
Figure 1: Shows a case of a 30-year-old male presented with two attacks of seizures associated with recurrent episodes of nausea and vomiting. On examination, there was no motor or sensory deficit, Glasgow coma score (GCS) was 15 with no fit or vomiting. A brain CT scan (A) was ordered and showed a left parietal AVM confirmed by MRA (B). DSA was performed to reveal exactly the location and characteristics of the feeding arteries and draining veins (C). Post-operatively, the patient’s GCS was 15 with no fit and only one episode of vomiting. Motor examination revealed a decrease in the initiation of movement; otherwise, the patient was improved. Post-operative CT was done and showed total excision of the AVM (D).
View Figure 1
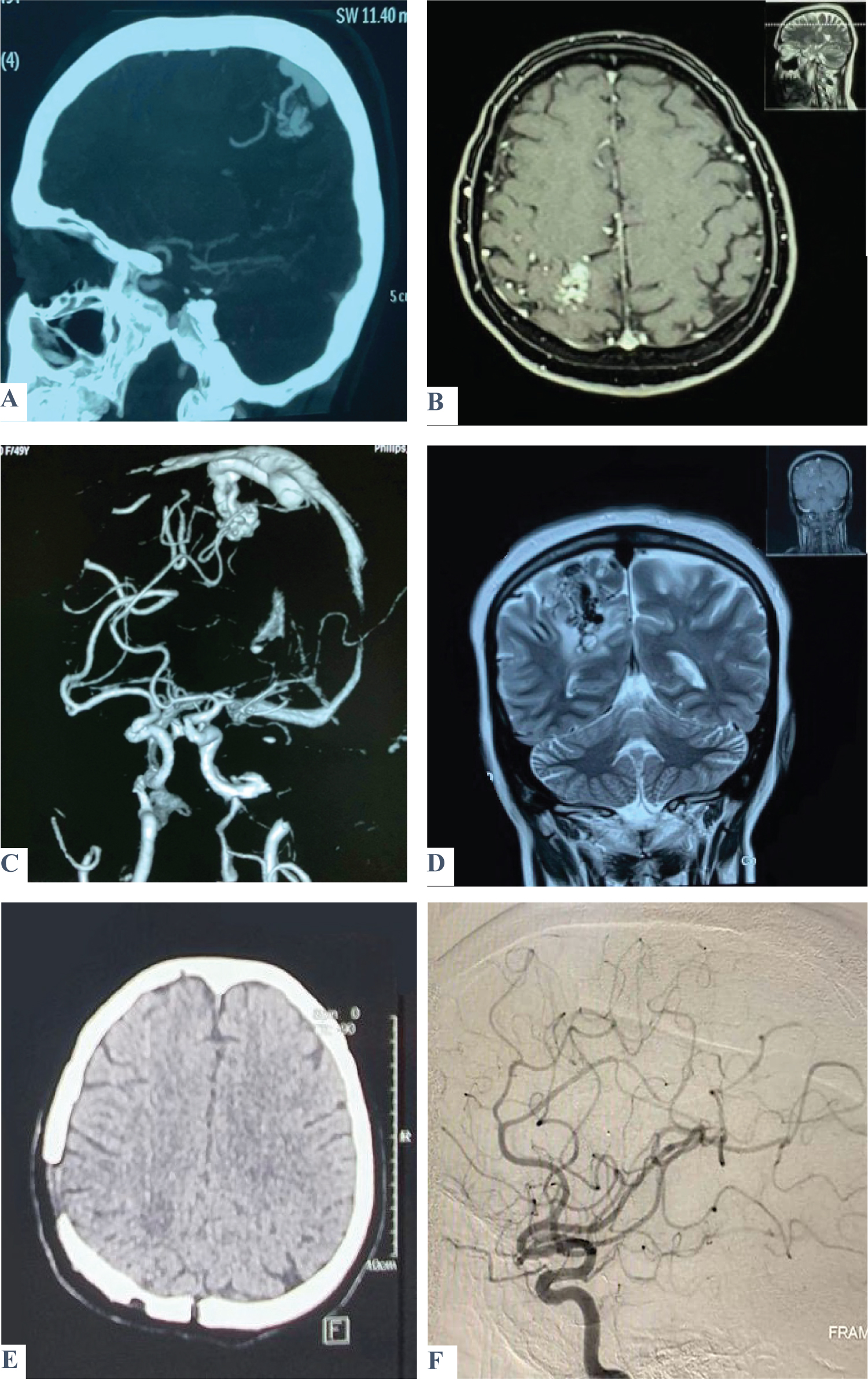 Figure 2: Shows a case of a 49-year-old female patient presented with three attacks of seizures. On admission, GCS was 15, with no fit, no vomiting, and no motor or sensory deficit. A brain CT scan (A) and MRI (B) show a heterogenous lesion within the posterior part of the Rt. parietal lobe involving the sensory area consistent with AVM, which was confirmed by MRA (C) and MRV (D). Post-operatively, GCS was 15 with no fit or vomiting. There was left-lower limb weakness but relieved on successive days. A postoperative brain CT scan and DSA revealed total excision of the lesion (E & F).
View Figure 2
Figure 2: Shows a case of a 49-year-old female patient presented with three attacks of seizures. On admission, GCS was 15, with no fit, no vomiting, and no motor or sensory deficit. A brain CT scan (A) and MRI (B) show a heterogenous lesion within the posterior part of the Rt. parietal lobe involving the sensory area consistent with AVM, which was confirmed by MRA (C) and MRV (D). Post-operatively, GCS was 15 with no fit or vomiting. There was left-lower limb weakness but relieved on successive days. A postoperative brain CT scan and DSA revealed total excision of the lesion (E & F).
View Figure 2
The Spetzler-Martin classification is the most widely accepted system for pre-operative AVM evaluation [1]. It has been extensively used because of its ease, simplicity, and practicality. Nevertheless, the classification can be misleading in some cases. One possible drawback of this system is that it lacks the ability to properly define the type of eloquent areas; consequently, patients with eloquent sensorimotor AVM are equal to patients with other eloquent AVM, for example, visual cortex AVM, and have the same grade [7].
Grade III AVM represents a dilemma and exemplifies the controversial paradigm of the Spetzler-Martin classification. It may include any combination of size, eloquence, and venous drainage. In the proposed 3-tier system by Spetzler-Ponce, Grade III AVMs remain separate as Class B and receive multimodality treatment [2]. Consequently, the management plan for grade III AVM is yet an unresolved problem, and for that reason, many papers have been published in the literature discussing various modalities and subclassifications for this grade [3,4,8,9].
For the grade III peri-Rolandic AVM, as in our case series, all the AVMs should involve an eloquent area (one point) and have superficial venous drainage (zero point); this obligates the size to be between 3-6 cm to receive two points and become grade III. The clinical presentation of this lesion is typically seizure, as in all our cases. The peri-Rolandic territory involves the pre- and post-Rolandic areas, which represent the motor and sensory cortex, respectively. In our case series, we found 3 motor and 5 sensory grade III AVMs, which represent sensory dominance, despite the small number of patients. We believe that peri-Rolandic areas are the most critical and eloquent areas that have diverse functional and clinical outcomes. A study found that sensorimotor eloquence would have a greater association with poor outcomes than the other eloquence subtypes [8], and although eloquent sensorimotor AVMs may receive the same Spetzler-Martin grade as an otherwise equivalent AVM of the visual cortex, the risk of a poor outcome may be higher with the former.
The management of grade III AVMs with an eloquent involvement is individualized and multimodal. The most common management options used are embolization and radiosurgery due to the perceived surgical risk [6]. Many authors support radiosurgical management since it is more appealing and essentially devoid of immediate treatment-related complications [4], while others go with endovascular management, which expands the ability to treat a wider range of AVMs [5]. However, in certain lesions, like peri-Rolandic AVMs, microsurgical resection is possible, especially for superficial, small-sized lesions. Indeed, microsurgery is still the most promising and ultimate method; it has been demonstrated to be superior in terms of overall risk reduction of future hemorrhage, primarily by means of obliteration rates nearing 100% [10-13]. In peri-Rolandic AVM, microsurgery is favorable and yields excellent AVM obliteration with minimal neurological morbidity in selected patients where the lesion is small in size and readily accessible through the cerebral fissures, which makes the resection smooth and straightforward [14].
The difficulty and risks involved with the surgical resection of grade III peri-Rolandic AVMs and the prognosis of these lesions are completely different. Unlike other types of eloquent AVMs, sensorimotor AVMs have worse outcomes and more neurological deficits after microsurgical resection [7]. However, most of these deficits are relieved in the long-term follow-up. In our case series, the majority of the patients (75%) developed postoperative deficits in the form of weakness and dysphasia, and a postoperative seizure was detected in two of the motor AVMs. Subsequently, on the long-term follow-up, these deficits were relieved, and the mRS score for all our patients was 1. This denotes that the frequency of disabling permanent neurological complications is nearly zero, which goes with the generally reported low complication rate of AVM microsurgery [15].
This study has a number of limitations, predominantly due to its retrospective nature. All the patients derived from a single center. The small number of included patients is the main limitation factor; It prevents accurate results and lacks the grand diversity of clinical presentation.
In our study, we believe there still exists a group of patients harboring grade III AVMs in the peri-Rolandic area for whom the microsurgical management is demanding and challenging, but promising and heartening.
Microsurgical management remains more effective in treating Spetzler-Martin grade III AVMs when the peri-Rolandic eloquent area is involved. This is due to the precise control and visualization provided by microsurgical techniques, which allow for safer resection of the AVM while minimizing damage to important brain functions.
The authors have no Conflict of interest and financial support to disclose.