Chronic rhinosinusitis is defined as the inflammation of mucosa of nose and paranasal sinus for at least 12 consecutive weeks of duration. Being one of the most common health problems worldwide, its pathogenesis is not well defined and there may be multiple aetiologic factors. Bacteria, fungi or viruses may be involved in some cases but there may be cases with no identifiable pathogenic organisms. As there is emerging drug resistance management of patients is also getting troublesome.
To find out the bacteria and fungi responsible for chronic rhinosinusitis and the antimicrobial susceptibility of the isolates.
A prospective cohort study of 100 cases of chronic rhinosinusitis without nasal polyposis as per rhinosinusitis task force definition, whose diagnostic nasal endoscopy done and specimens were obtained in which aerobic, anaerobic, fungal cultures were performed and sensitivity was obtained. All the patients were managed as per the protocol for treatment.
Most common bacteria isolated was Staphylococcus aureus (34%) followed by Pseudomonas aeruginosa (12%), Enterococcus faecalis (3%), Escherichia coli (2%), Proteus mirabilis (1%) and Citrobacter spp. (1%). Staphylococcus aureus was most sensitive to cefixime (63.66%) followed by ciprofloxacin (42.42%), gentamicin (39.39%) and erythromycin (36.46%). Pseudomonas aeruginosa was most sensitive to ciprofloxacin (66.7%) followed by cefixime (33.3%) and erythromycin (25%). Enterococcus faecalis showed sensitivity to erythromycin and gentamicin (66.7%). Escherichia coli was most sensitive to co-trimoxazole (100%) followed by ciprofloxacin and chloramphenicol (50%). Most sensitive antibiotic was found to be cefixime (52.83%), followed by ciprofloxacin (50.94%), erythromycin (32.1%), gentamicin (30.18%), and co-trimoxazole (15.09%) where as amoxicillin + clavulonic acid (5.6%) and methicillin (13.2%) were found to be least sensitive. Three percent (3%) showed fungal hyphae on KOH wet mount.
Common organism causing chronic rhinosinusitis is Staphylococcus aureus followed by Pseudomonas aeruginosa and most sensitive antibiotics were cefixime and ciprofloxacin. Commonly used antibiotics like amoxicillin-clavulonic acid is losing effectiveness due to the emerging drug resistant bacterial strains.
Chronic rhinosinusitis, Clinico microbiological profile, Antibiotic sensitivity, Drug resistance
Chronic rhinosinusitis (CRS) is defined as the inflammation of mucosa of nose and paranasal sinus for at least twelve consecutive weeks of duration [1]. Rhinosinusitis task force by American Academy of Otorhinolaryngology and Head and Neck Surgery defined rhinosinusitis as the presence of two major symptoms or one major and two minor symptoms [2,3]. Major symptoms include facial pain/pressure, facial congestion/fullness, nasal obstruction/blockage, nasal discharge/purulence, postnasal drip, hyposmia/anosmia or purulence on nasal examination. Minor symptoms include headache, fever (non-acute), halitosis, fatigue, dental pain, cough, ear pain/pressure/fullness [4]. Nearly 83% of patients presenting with an acute upper respiratory tract infection have bacterial rhinosinusitis [3,5]. An extrapolated data shows that in India 136,657,953 individuals out of 1,065,070,607 have chronic rhinosinusitis [6].
Etiology of chronic rhinosinusitis includes ostiomeatal obstruction, allergies, polypi, dental diseases and occult or subtle immunodeficiency states, which lead to inflammation of nose and paranasal sinus [7,8]. It is diagnosed by symptoms mentioned by Rhinosinusitis task force together with either endoscopic signs of oedema/mucosal congestion, mucopurulent discharge or polypi coming from middle meatus and/or computed tomography changes like mucosal changes within ostiomeatal complex and/or sinuses [3,4]. Common organisms are Staphylococcus species (55%) and out of this Staphylococcus aureus is the major organism (20%). Some studies have shown a high prevalence of Enterobacteriaceae organisms, anaerobes, Gram-negative bacteria and fungi [9-13]. However no significant etiology for CRS has been identified to date. So a definitive microbial profile responsible for disease manifestation remains controversial. Healthy sinuses may harbor a diverse assemblage of microorganisms and alteration in the kinds and quantities of these microbes may play a role in the pathogenesis of CRS. Treatment includes medical or surgical therapy. Medical therapy mainly includes the use of antibiotics. Antibiotic resistance is an emerging problem now-a-days, which is becoming a roadblock in providing effective treatment. Extended spectrum betalactamases (ESBL) and methicillin resistant staphylococcus aureus (MRSA) strains represent a major threat among MDR bacterial isolates. Study conducted by Rezai, et al. showed increased role of MRSA and ESBL producing bacteria in chronic sinusitis. 47.61% of staphylococcus strains were MRSA and 33.33% of enterobacteriacea isolates were ESBL producing bacteria [11]. In a study conducted in US, Rujuvanej, et al., examined 10,387 positive intranasal culture samples over a period of two decades from 1990 to 2010 and it was found that Staphylococcus aureus was seen in 800 (7.7%) and out of which 110 (1.06%) were methicillin resistant staphylococcus aureus (MRSA) and it showed a statistically significant increasing trend for MRSA sinusitis [14].
As the drug resistance in microorganisms is an emerging problem and it varies from time to time, hence, the present study aims to find out the organisms causing chronic rhinosinusitis and their drug sensitivity pattern in a study group without nasal polyposis.
This prospective observational study was conducted in the Department of ENT, in collaboration with Department of Microbiology, Government Medical College Hospital, Sector- 32 Chandigarh, India. One hundred (100) patients clinically diagnosed as chronic rhinosinusitis as per rhinosinusitis task force definition [1] irrespective of age and sex were evaluated in the study. Those who received antibiotic in the last one week of presentation, patients with nasal polyposis and immunocompromised patients were excluded. Patients were subjected to diagnostic nasal endoscopy under local anaesthesia using 4% xylocaine solution. Under all aseptic precautions, samples were taken under endoscopic guidance (nasal endoscope of 0°, 4 mm diameter for adults and 2.7 mm diameter for children of Karl-Storz/Stryker) from middle meatus area, thus avoiding any risk of contamination within the nasal cavity. Immediately swabs were placed in two sterile test tubes for bacterial and fungal studies. Test tubes containing the specimen were labelled and taken to Microbiology laboratory and were studied for bacterial and fungal culture and sensitivity.
• For aerobic culture swabs were inoculated on blood agar, MacConkey's agar and brain heart infusion broth (BHIB) and incubated for 24 hours at temperature of 37 °C. If no growth is seen on agar or no turbidity in BHIB after 24 hours, the plates and BHIB was further incubated for another 24 hours and examined as above. If still no growth is seen, then the culture was reported as sterile. For anaerobic culture, sample was inoculated in Robertson Cooked Meat media (RCM) and was incubated for 24 hours at 37 °C. Results were recorded after 48 to 72 hours of incubation. Then the individual colonies were inspected and aerotolerance test was done for each colonies. Those organisms, which failed to grow aerobically, were considered as anaerobes. Pure culture isolates were identified by standard conventional methods.The culture isolates were studied for their sensitivity to the antibiotics namely - ciprofloxacin, gentamicin, co-trimoxazole, cefixime, amoxicillin + clavulanic acid, chloramphenicol, methicillin, erythromycin, polymyxin B, neomycin and ofloxacin. Kerby-Bauer disc effusion method was used for this purpose. Fungal examination was also done.
• KOH wet mount: A small amount of specimen was examined after partial digestion with 10-20% KOH as slide and tube KOH wet mounts. It is used to detect characteristic presence of fungal elements.
• Fungal culture: The specimen was inoculated under sterile conditions to Sabaroud dextrose agar tube slants with chloramphenicol, gentamicin and cyclohexamide and on another set of Sabaroud dextrose agar with chloramphenicol and gentamicin and was incubated on each at 37 °C and at 22 °C. The specimen was also inoculated in Brain Heart Infusion Broth (BHIB) and blood agar and was incubated at 37 °C. The culture was examined for growth daily for the first week and twice a week for the next three weeks before reporting as sterile.
• Identification of fungal isolates: The fungal isolates so obtained was identified on the basis of gross morphological features and microscopic examination by Lactophenol cotton blue (LCB) mount and assessed by standard conventional mycological methods for identification of fungal species. The culture isolates were studied for their sensitivity to amphotericin, itraconazole, voriconazole, flucytosine, fluconazole, caspofungin and ketoconazole.
• All the patients were managed as per the protocol for treatment of chronic rhinosinusitis by the treating consultant. The reports were collected and entered in records and the patient's treatments were modified as per the report of culture and sensitivity.
Written informed consent was obtained from all patients, and the study protocol was approved by the Institutional Research and Ethical Committee. The data was compiled and statistically analysed to present the results in the form of numbers and percentages. Bacteriological profile and fungi profile were compared according to gender and age by using student t-test and analysis of variance (ANOVA) technique. Normal test of proportions was also applied for testing proportions of cases in different sub groups.
Males constituted majority of study population (64%). Out of total 60% resided in urban area and 40% from rural area. Most of the patients were between the age group of 20-29 years (35%) followed by 30-39 years (26%), 40-49 years (17%), more than or equal to 50 years (16%) and 10-19 years (6%). Baseline values are depicted in Table 1 and Table 2.
Table 1: Baseline values for gender. View Table 1
Table 2: Baseline values for age. View Table 2
Seventy patients (70%) were found to have nasal obstruction. Twenty six patients (26%) presented with right sided nasal obstruction, nineteen patients (19%) with left sided nasal obstruction and twenty five patients (25%) with bilateral nasal obstruction. Seventy six out of hundred (76%) patient had nasal discharge. Thirty four out of hundred (34%) had yellowish discharge and 42% had whitish discharge. Duration of symptoms vary from 1-12 years. Thirty six have complaints for 2-4 years while less than 2 years duration of complaint is present in 31%. As duration increases number of patients decreases gradually, showing only 2 (2%) patients in more than 10 years group. Forty two patients had mucoid discharge, 32 patients had mucopurulent discharge and 26 patients showed no discharge. Twenty two percent (22/100) presented with nasal bleed. Fifteen out of hundred (15%) patients showed facial congestion/fullness. Hyposmia/anosmia as a presenting complaint was seen in 21% (21/100) of patients. Fourteen patients (14%) presented with facial pain/fullness. Post-nasal discharge was the presenting complaint in 36/100 (36%) of patients. Although headache is common, only 26/100 (26%) of patients presented with headache. Ten percent presented with fever and 9% with halitosis, cough, ear pain/fullness. 11% had fatigue, 8% of patients had dental pain and 22% had history of allergic symptoms (Figure 1).
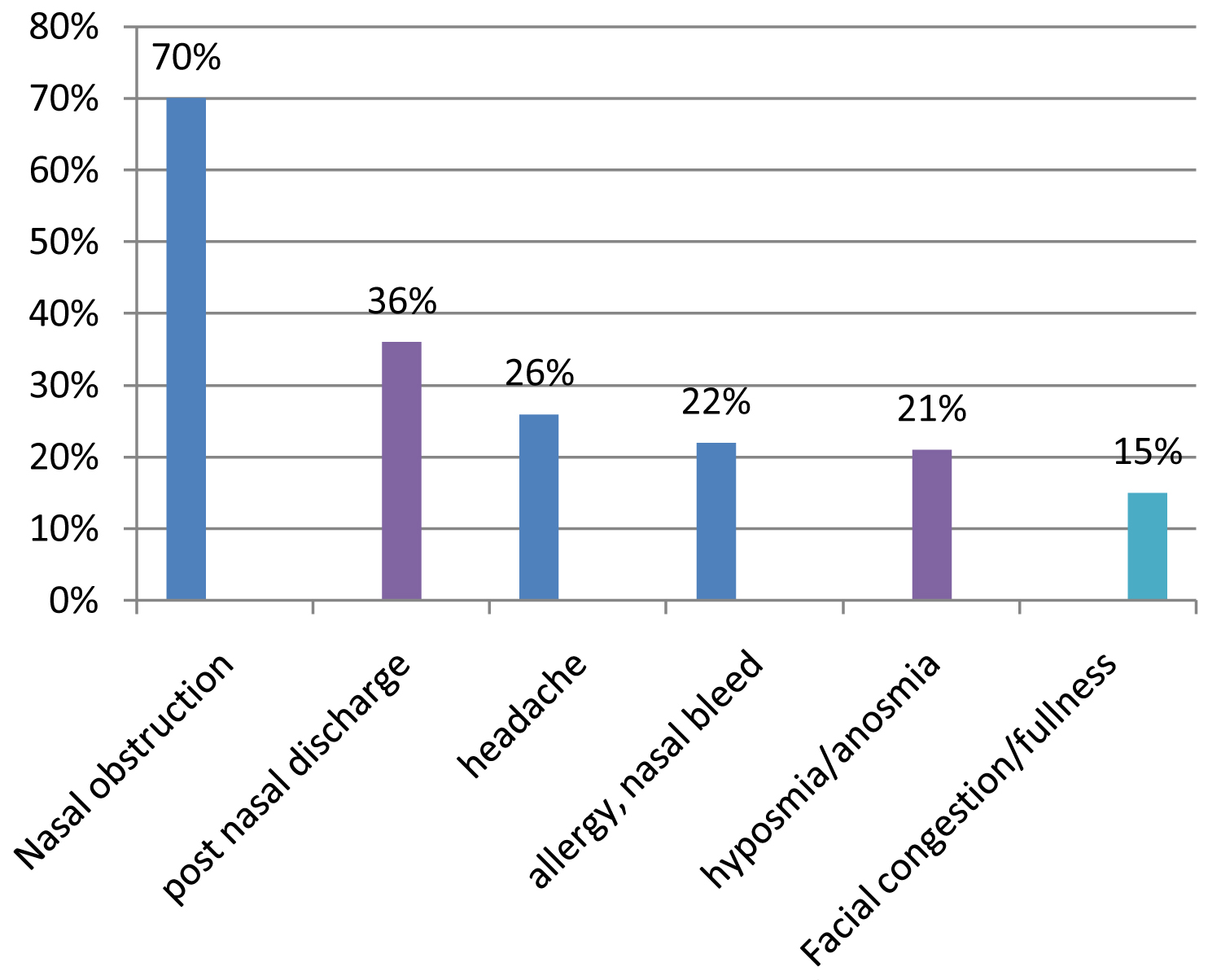 Figure 1: Major presenting symptoms.
View Figure 1
Figure 1: Major presenting symptoms.
View Figure 1
Seventy out of hundred (70%) of patients on examination showed deviated nasal septum. On nasal endoscopy 11% patients showed mucopurulent discharge in nasopharynx and 9% showed mucoid discharge. Eight patients (8%) showed adenoid hypertrophy. In 72% of patients nasopharynx were clear (Figure 2). On nasal endoscopy middle meatus showed mucoid discharge in 66% patients and remaining 34% showed mucopurulent discharge. Non contrast computed tomography of nose and paranasal sinus was done in 56% patients. Homogenous soft tissue density was seen in maxillary sinus (29%), followed by ethmoid sinus (27%), followed by sphenoid sinus (5%) and frontal sinus (8%). Two patients showed clear scan. Seventy seven percent showed ostiomeatal obstruction.
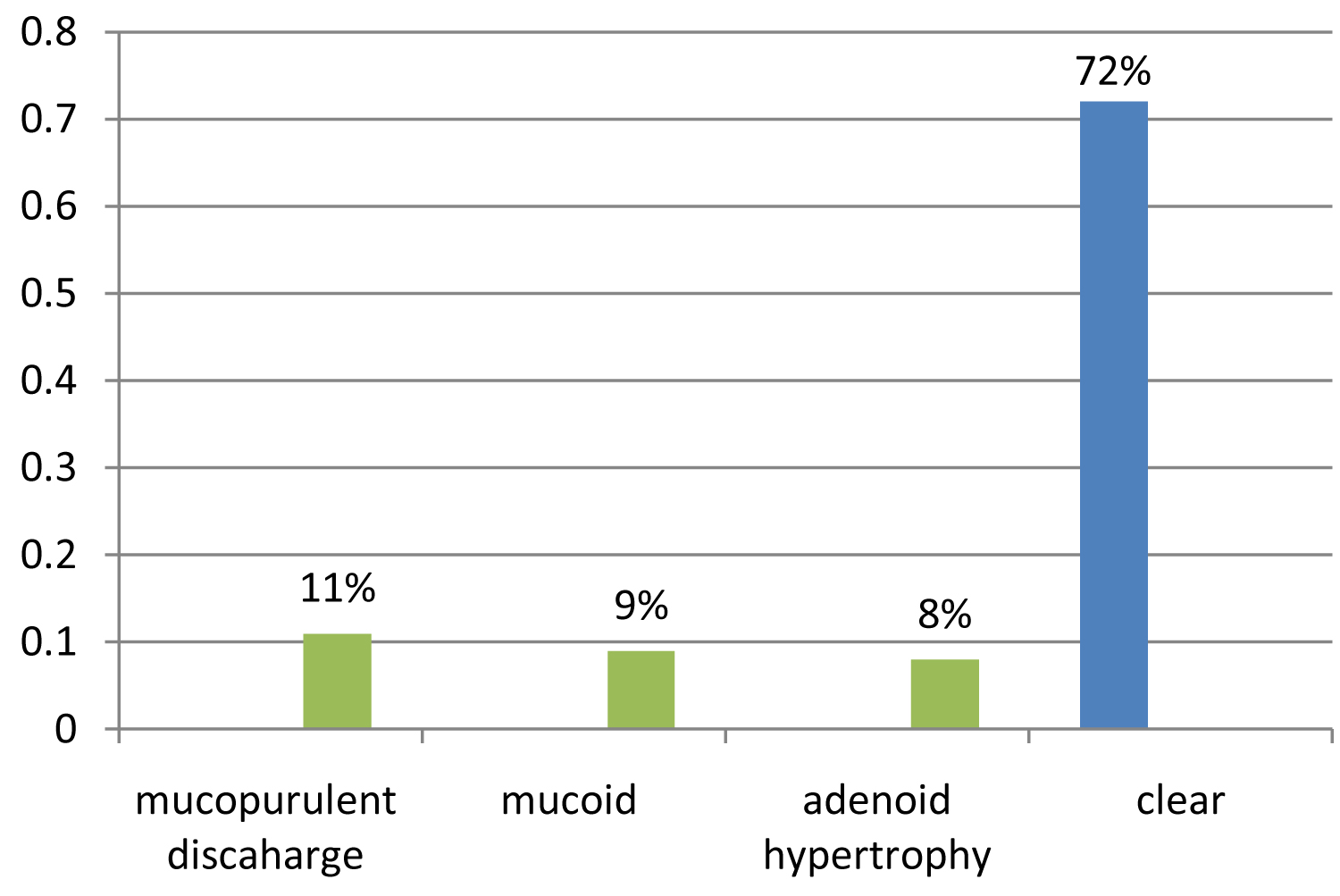 Figure 2: Nasal endoscopy findings.
View Figure 2
Figure 2: Nasal endoscopy findings.
View Figure 2
Forty seven (47%) show gram-positive cocci, followed by gram-negative bacilli (24%) and gram-negative cocci (5%). Most common bacteria isolated is Staphylococcus aureus and is seen in 34% of samples followed by Pseudomonas aeruginosa in 12% of samples followed by Enterococcus faecalis (3%), Escherichia coli (2%), Proteus mirabilis (1%) and Citrobacter spp. (1%) (Figure 3).
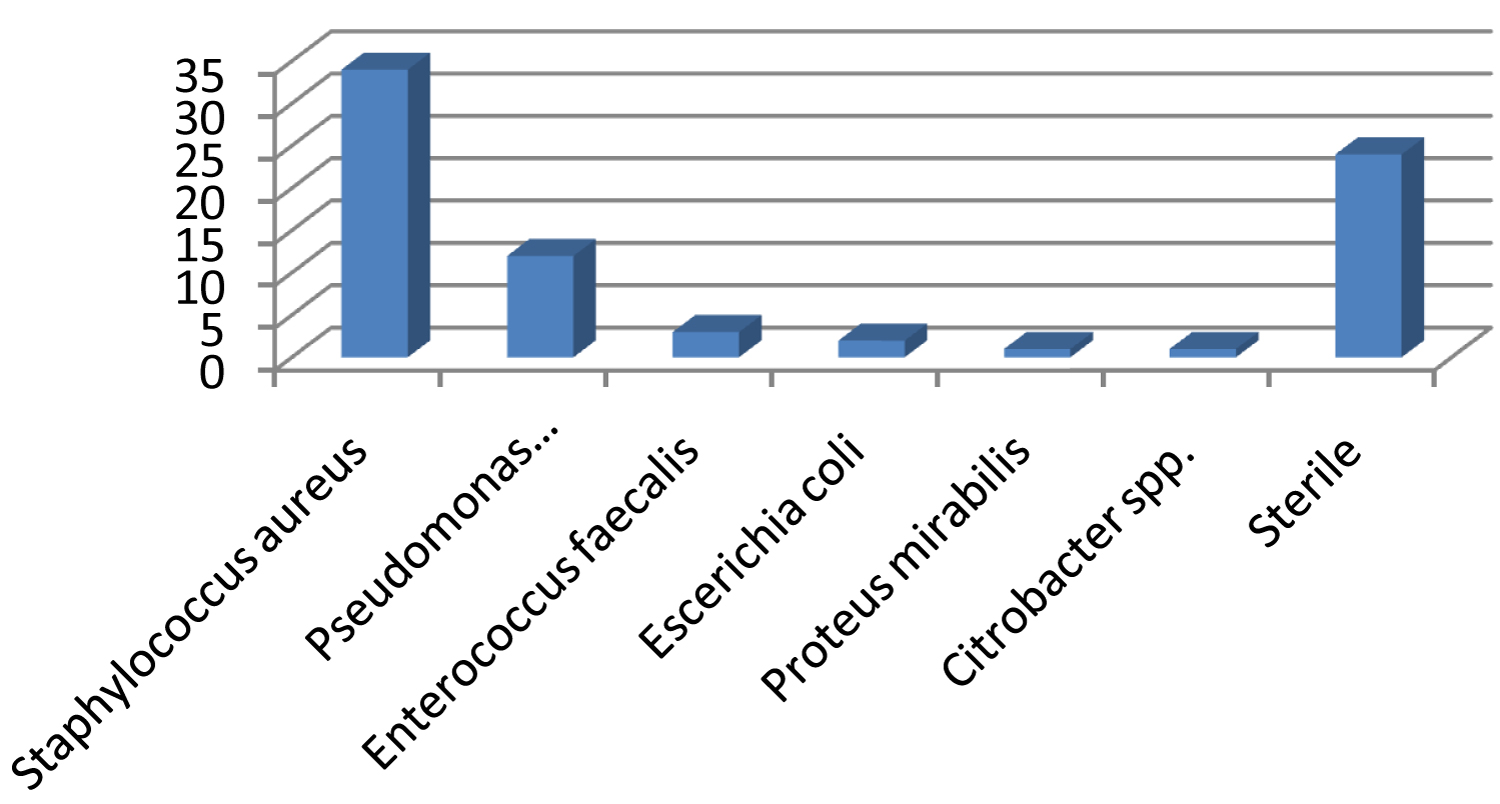 Figure 3: Culture results in study group.
View Figure 3
Figure 3: Culture results in study group.
View Figure 3
While comparing clinical features with organisms isolated from study group, mucopurulent nasal discharge, nasal obstruction, PND , hyposmia was more associated with staphylococcus isolates whereas halitosis, facial pain, dental pain along with mucopurulent discharge were common with pseudomonas aeruginosa isolates.
Most sensitive antibiotic against Staphylococcus aureus as per the study is cefixime showing 63.66% are sensitive to cefixime followed by ciprofloxacin showing 42.42% sensitivity, gentamicin showing 39.39%, erythromycin showing 36.46%, methicillin showing 21.21%, co-trimoxazole 15.15% and chloramphenicol 12.12%, least sensitive were amoxicillin + clavulonic acid (3.03%), polymixin, neomycin and ofloxacin (Figure 4).
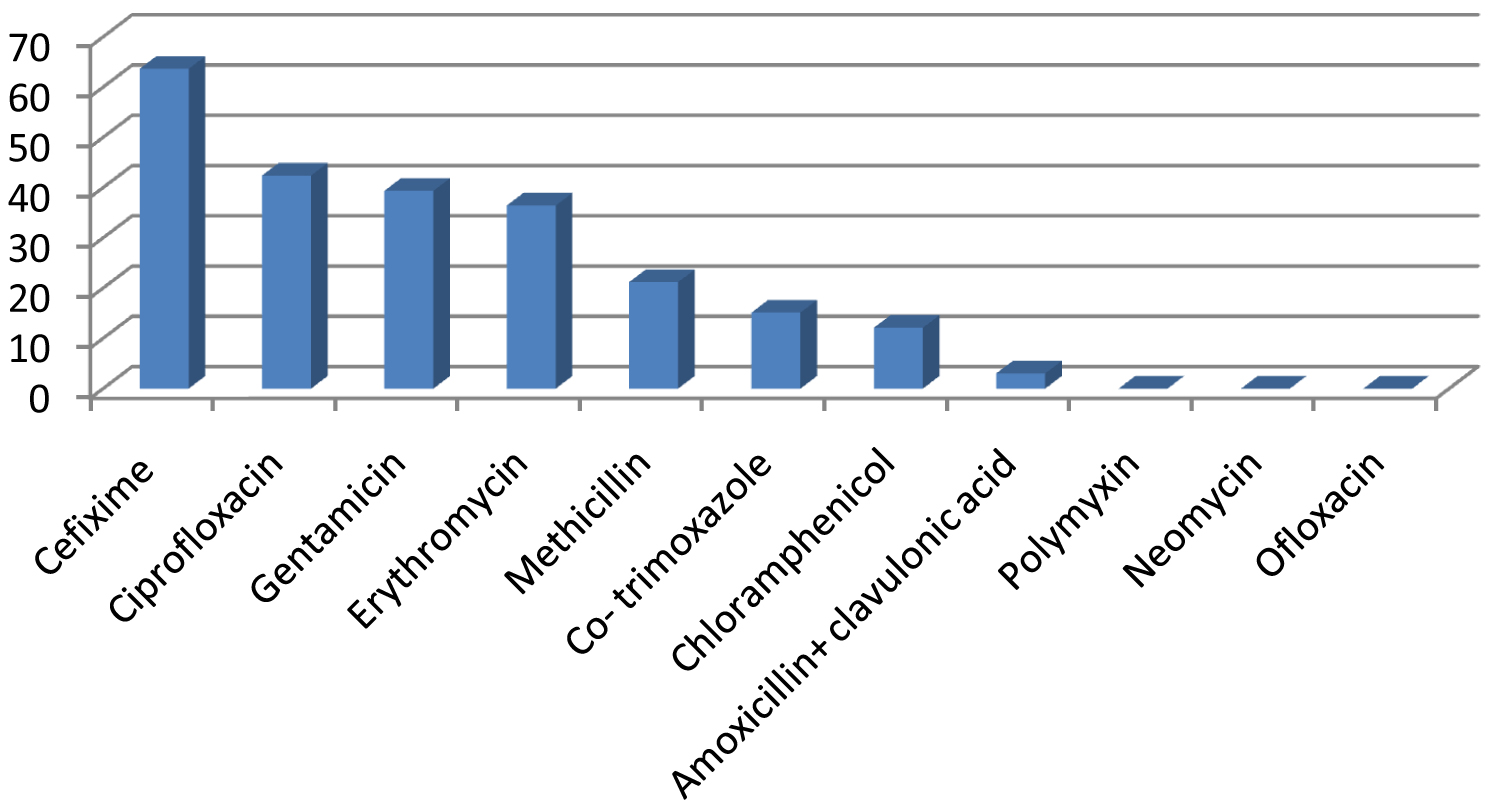 Figure 4: Sensitivity pattern of Staphylococcus aureus in study group.
View Figure 4
Figure 4: Sensitivity pattern of Staphylococcus aureus in study group.
View Figure 4
Most of the pseudomonas aeruginosa were sensitive to ciprofloxacin (66.7%) followed by cefixime (33.3%), erythromycin (25%), polymyxin (16.7%) and amoxicillin + clavulonic acid (8.3%). Organisms were resistant to methicillin, gentamicin, erythromycin, chloramphenicol, neomycin and ofloxacin (Figure 5).
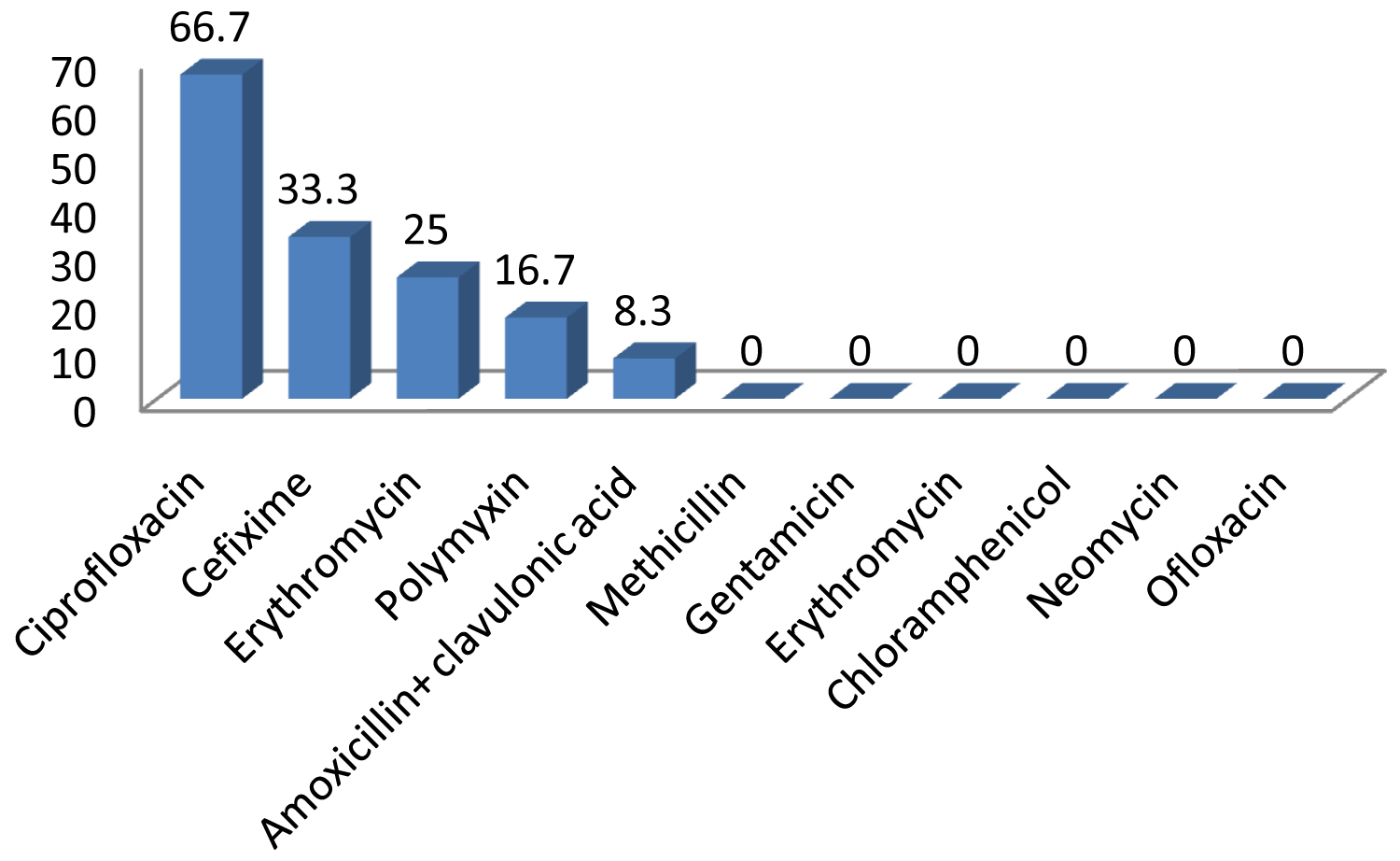 Figure 5: Sensitivity pattern of Pseudomonas aeruginosa in study group.
View Figure 5
Figure 5: Sensitivity pattern of Pseudomonas aeruginosa in study group.
View Figure 5
On observing overall sensitivity pattern most sensitive antibiotics are cefixime showing 52.83%, followed by ciprofloxacin showing 50.94%, erythromycin (32.1%), gentamicin (30.18%), co-trimoxazole (15.09%), methicillin (13.2%), chloramphenicol (11.32%). Least sensitive are amoxicillin + clavulonic acid (5.6%), polymyxin (3.8%). Neomycin and ofloxacin are not sensitive (Figure 6).
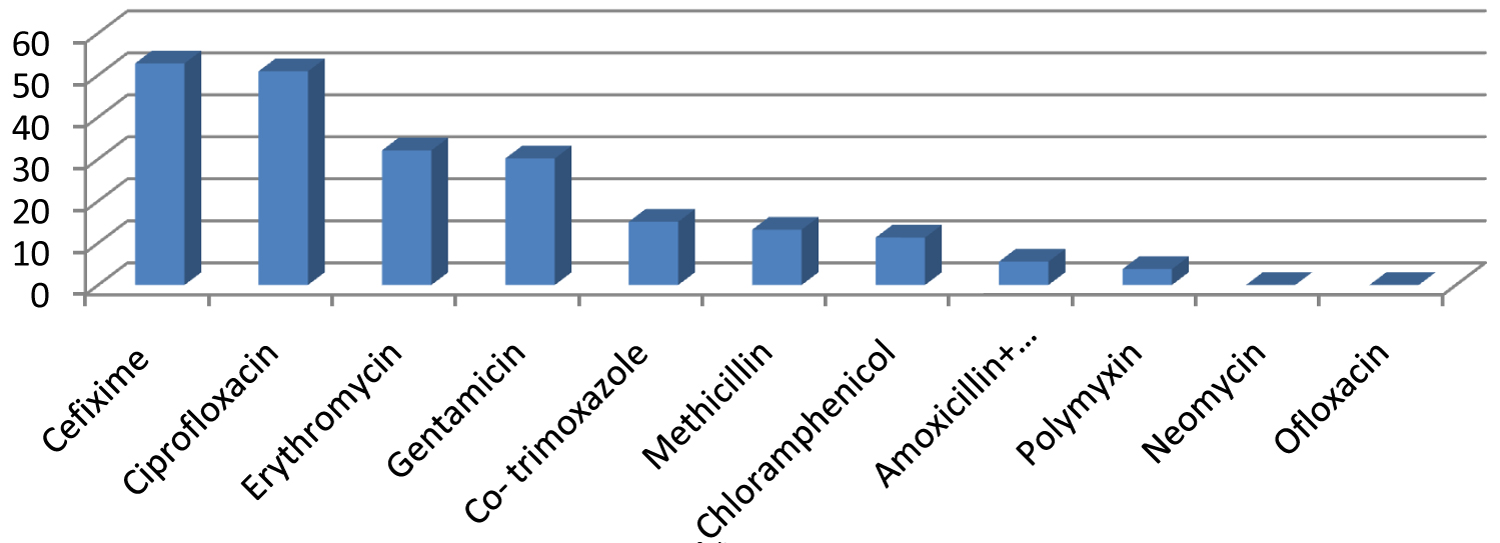 Figure 6: Overall drug sensitivity profile in study group.
View Figure 6
Figure 6: Overall drug sensitivity profile in study group.
View Figure 6
Chronic rhinosinusitis is the inflammation of nose and paranasal sinus which last at least 12 weeks [1,3]. It is one of the most common health problems in India [6].
In our study, age of presentation varied from 11 to 76 years, with mean of 35.80 and standard deviation of 13.845. This is comparable with the observations of Brook, who reported mean age as 47 years ranging from 6 to 67 years [15]. The observations of Rezai, et al. who reported the average age was 34.2 ± 11 and range from 8 to 68 years is also similar [16].
Out of one hundred patients, sixty four were male (64%), whereas, thirty six were female (36%). The male to female ratio is 1.8:1. Study done by Erkan, et al. also showed a male predominance of 2.15:1 [11]. It is comparable with the observations of Puglisi, et al. who reported male to female ratio of 1.11:1 [17]. The relatively higher involvement of males in outdoor activities increasing their exposure to irritants, higher prevalence of smoking and hesistance of females in the social setting like India to seek medical care may be the reasons for this observation.
Geographically, in our study, patients belonging to urban area were found to be more. Sixty out of one hundred (60/100) patients came from urban area (60%) and forty, out of one hundred patients (40/100) came from rural area (40%), showing urban to rural ratio of 1.5:1. This is in agreement with the study conducted by Farhani, et al. who also stated urban to rural ratio as 1.34:1 [18].
Seventy six percent of patients in our study, presented with nasal discharge (76/100). These observations are comparable with the study done by Fergusen, et al. who reported 56% patients with nasal discharge [19]. Thirty four patients in our study had yellowish nasal discharge, whereas, it was whitish in 42% patients.
Next common symptom in the present study was nasal obstruction. Out of the 100 patients, 70 patients presented with nasal obstruction. Twenty six percent (26%) of patients showed right nasal obstruction, nineteen percent (19%) had left nasal obstruction and twenty five percent (25%) reported bilateral nasal obstruction. Twenty one percent (21%) of patients presented with hyposmia/anosmia. It is similar to the observations reported by Irfan, et al. who found out 76% of patients with nasal obstruction and 21% patients with hyposmia/anosmia [20].
Thirty six percent of patients (36/100) in our study showed postnasal discharge which is comparable to the observations by Madani, et al. who reported postnasal discharge in 40% patients [21].
The prevalence of Gram-positive organism was found to be high. Forty seven patients (47%) showed Gram-positive bacteria and twenty nine patients (29%) showed Gram negative bacteria. This observation relates with the study done by Roumbax, et al. who reported 56.6% Gram positive bacteria and 32% Gram negative bacteria [10].
Staphylococcus aureus was the most common organism in our study. Thirty four patients (34%) were found affected by Staphylococcus aureus. It is comparable with the observations by Doyle and Woodham who reported as 33% [20] and Irfan, et al. reported as 46% [21] (Table 3).
Table 3: Comparing microbiological profile of our study with Doyle and Woodham [21], Busaba, et al. [22] and Irfan, et al. [20]. View Table 3
Second most common organism in our study was Pseudomonas aeruginosa. Twelve percent (12%) culture isolates were found to be positive for Pseudomonas aeruginosa. Similar observations were seen by Busaba, et al. (5%) [22]. Another study done by Finegold, et al. also reported 11% incidence of Pseudomonas aeruginosa [23].
Enterococcus faecalis was found in three patients. Doyle and Woodham, also observed 1.1% of culture to be affected Enterococcus faecalis [21]. Rombaux, et al. reported 0.9% cultures with Enterococcus faecalis [10].
In our study only two patients showed Escherichia coli as the causative organism. A study done by Finegold, et al. showed prevalence of Escherichia coli in 1.1% specimens [23]. Rombaux, et al. showed a prevalence of 9.5% [10]. Proteus mirabilis was found in only one patient i.e.1% in our study. Finegold, et al. also reported Proteus mirabilis with prevalence of 1.4% in their study [23].
One percent of patient showed Citrobacter spp. in our study. Similar observations reported by Irfan, et al. showed 1% in their study [20].
Fungal hyphae were found positive in three out of hundred patients' i.e.3%. Similar observations were stated by Doyle & Woodham (1.1%) [21]. Busaba, et al. showed no presence of fungi [22]. This concludes that the role of fungi in chronic rhinosinusitis is not very significant.
Most common isolate in our study was Staphylococcus aureus. The sensitivity pattern of Staphylococcus aureus widely varies. Most sensitive antibiotic was cefixime showing sensitivity of 63.66% followed by ciprofloxacin showing 42.42% sensitivity, gentamicin 39.39%, erythromycin 36.46%, methicillin 21.21%, co-trimoxazole 15.15%, and chloramphenicol 12.12%. The isolates showed low sensitivity to amoxicillin-clavulanic acid (3.03%), polymixin, neomycin and ofloxacin. This is comparable with the study done by Puglisi, et al. who observed 52% sensitivity to erythromycin. In their study penicillin showed only 26% sensitivity [17]. Similar results were showed by Rezai, et al. who reported 40% sensitivity of ciprofloxacin followed by gentamicin (20%) [16]. This result needs more concern as amoxicillin-clavulanate is one of the most frequently used drug throughout.
Pseudomonas aeruginosa, which was the second most common isolate in our study was most sensitive to ciprofloxacin (66.7%), followed by cefixime (33.3%), erythromycin (25%), polymixin (16.7%) and amoxicillin + clavulanic acid (8.3%). It is comparable to the study done by Farahani, et al. who reported maximum sensitivity of ciprofloxacin (76.7%) and chloramphenicol (41.7%) [18].
In our study Pseudomonas aeruginosa was found resistant to methicillin, gentamicin, erythromycin, chloramphenicol, neomycin and ofloxacin. Farahani, et al. also reported that most resistant antibiotic was penicillin. In their study 91.7% organisms were resistant to penicillin followed by ampicillin (83.4%).
Thus, it is clear from the present study that bacterial infection is a major etiological factor in chronic rhinosinusitis. The sensitivity profile of these bacteria varies a lot and is being affected by the emergence of drug resistance. Proactive instead of reactive methods are always more valuable. Proper antibiotic stewardship is the need of the hour. Therefore, a more rational use of antibiotics is needed to be implied in the treatment of patients suffering from chronic rhinosinusitis to obtain the maximal effect.
From the present study "Clinico-microbiological evaluation of chronic rhinosinusitis in North India" it can be concluded that the most common organism causing chronic rhinosinusitis is Staphylococcus aureus followed by Pseudomonas aeruginosa. Most sensitive antibiotics in chronic rhinosinusitis were cefixime and ciprofloxacin. Commonly used antibiotics like amoxicillin-clavulonic acid is losing effectiveness due to the emerging drug resistant bacterial strains.
There is no any conflict of interest.
Current study involves human participants.
There is no any funding source for this study.
This study was approved by the Ethical committee of Government Medical College and Hospital Chandigarh.
Informed and written consent was taken from all patients before enrolling them in the study.
This study would not have been achieved without the co operation of microbiology department GMCH Chandigarh. We sincerely appreciate their contribution.
The authors declare that they have no conflict of interest.