This study examined the effect of adding a high dose of resistant starch (RS) in plain muffins on human glycemic response in sedentary and abdominally obese individuals. A total of 8 participants were randomly assigned to two sequences of treatments (AB, BA) using a 2 × 2 randomized cross-over design. The treatment effect was tested with a muffin that contained 75g of digestible carbohydrates and 30g of RS as treatment condition (TRT, B), while the control effect was tested using a 75-g oral glucose solution as a control condition (CON, A). Linear mixed models were used to test the effects of sequence (AB vs. BA), treatment (TRT vs. CON), and time periods on 2-h glucose values and area under the curve (AUC) after adjustment for covariates (i.e., age, sex, and race). The 2-h postprandial glucose AUC was significantly lower in the TRT than the CON (12.5 vs. 15.6 mmol/L•h, P = 0.002). The glucose levels were also significantly lower in the TRT than did the CON at 30 minutes (6.6 vs. 8.3 mmol/L, P = 0.001), 60 minutes (6.6 vs. 8.5 mmol/L, P = 0.004), and 90 minutes (6.3 vs. 8.1 mmol/L, P = 0.003), respectively. The 2-h postprandial insulin AUC was also significantly lower in the TRT than the CON (P < 0.001). Based on the 2-h glucose incremental AUC values, the RS-supplemented muffins' calculated glycemic index was 48 (glucose = 100). This study suggests that adding 30g of RS supplementation decreased the glycemic index of muffins in sedentary and abdominally obese adults.
Resistant starch, Postprandial glucose, Sedentary, Obesity
Dietary fiber intake is associated with a reduced risk of coronary heart disease, stroke, colorectal cancer, and all-cause mortality [1-3]. Dietary fiber intake also improves glucose metabolism with a lower risk of developing type 2 diabetes [1,4-8]. The current public health concern is that an average fiber intake in the US population (16.2 g/day) is below the dietary guidelines' recommendations for Americans [9-13]. A national survey showed that less than 5% of US adults meet the daily fiber intake recommendations [10]. Exploring innovative and practical approaches to increasing fiber intake in an everyday western diet is necessary as a glycemic control strategy.
Dietary fiber intake is associated with a reduced risk of coronary heart disease, stroke, colorectal cancer, and all-cause mortality [1-3]. Dietary fiber intake also improves glucose metabolism with a lower risk of developing type 2 diabetes [1,4-8]. The current public health concern is that an average fiber intake in the US population (16.2 g/day) is below the dietary guidelines' recommendations for Americans [9-13]. A national survey showed that less than 5% of US adults meet the daily fiber intake recommendations [10]. Exploring innovative and practical approaches to increasing fiber intake in an everyday western diet is necessary as a glycemic control strategy.
Currently, the effect of a high dose of RS on GI remains less clear. Previous research has limited testing the acute effect of a low dose of RS on GI (2 to 11 g per serving) [16,20,21]. The US dietary guidelines recommended 14g of dietary fiber intake per 1000 kcal of energy intake [9]. Given that a dose-responsive relationship between RS and GI [16], we hypothesized that adding 30 g of RS, which is sufficient to meet the daily recommended dietary intake, will induce a further reduction in the GI of starch-containing food. Adding a high dose of RS to typical western food can be a practical method for the general population to meet the dietary guidelines' daily fiber intake [22]. This study investigated whether adding a high dose of RS in plain muffins (30 g of RS per muffin) lowers postprandial glucose levels in sedentary and abdominally obese individuals.
A total of eight study participants, who were sedentary and abdominally obese, were recruited by fliers and online posts from the Downtown Phoenix area. All participants provided written informed consent before study participation. Inclusion criteria comprised those who were 20 to 60 years of age, had a large waist circumference (men ≥ 102 cm, women ≥ 88 cm), and were not engaged in regular exercise. Exclusion criteria included those with RS allergy, diabetes, other metabolic disorders, celiac disease, antihyperglycemic medication use, a systolic blood pressure > 160 mmHg, or a diastolic blood pressure > 100 mmHg, or pregnant women.
We conducted a 2 × 2 randomized crossover trial to investigate the effect of RS-supplemented muffins on postprandial glucose responses in sedentary and abdominally obese individuals. All eligible participants were randomly assigned to two sequences of treatments (AB or BA). Participants in the AB sequence received a 75-g oral glucose solution in the first experiment condition (CON, A). In the second experiment condition (TRT, B), these participants received a muffin that contained 75 g of digestible carbohydrates plus 30 g of RS. Participants in the BA sequence underwent the two conditions in reverse order. There was a one-week washout period between treatments. The randomized sequence number was generated by an online program. The treatment allocation was concealed using electronically sealed envelopes. Outcome assessors were blinded to the randomization.
The recipe of the muffins was based on the national nutrient database for standard reference issued by the USDA. The nutrition facts of the muffins were listed in Table 1. The RS's source was Hi-Maize 260 (Honeyville, Rancho Cucamonga, CA), which contained 60% of RS and 40% digestible starch. The muffins were baked at 350 ℃ in the oven. The baked muffins were stored in a refrigerator and were heated in the microwave before serving. The glucose solution contains 75 g of anhydrous glucose (Glucola, Azer Scientific, Morgantown, PA).
Table 1: Nutrition facts of muffins. View Table 1
All participants were instructed to attend the experiment session after a 9-hour overnight fast. Each participant arrived at our lab between 8:00 and 9:00 AM. Upon arrival, anthropometrics were measured, including height, weight, and waist circumference. Resting blood pressure was measured after 5 minutes of rest using an automatic sphygmomanometer. After collecting baseline blood samples for plasma glucose and serum insulin measurement, we provided the participants with an RS-supplemented muffin or a bottle of glucose solution based on their randomized assignment. The glucose solution was consumed within 3 minutes, and the muffin was consumed within 10 minutes.
Plasma glucose and serum insulin were measured every 30 minutes. Blood samples were collected at baseline, 30 minutes, 60 minutes, 90 minutes, and 120 minutes. Plasma glucose values were analyzed using the in vitro hexokinase method. Serum insulin was measured by radioimmunoassay. GI was calculated as the 2-hour postprandial incremental AUC divided by the reference (glucose) AUC and multiplying the value by 100. All GIs that appeared in this article were based on the glucose reference. Insulin sensitivity was calculated using the regression model developed by Stumvoll, et al. [23]. The palatability of muffins was evaluated immediately after consuming muffins using the 9-point like-dislike scale developed by Peryam and Pilgrim [24]. The scale included "dislike extremely," "dislike very much," "dislike moderately," "dislike slightly," "neither," "like slightly," "like moderately," "like very much, and"like extremely."
Descriptive statistics were computed for anthropometrics, blood pressures, and blood glucose profiles. The normality assumptions were tested using the Shapiro-Wilk tests. Linear mixed models (LMM) were used to test the effects of sequence (AB vs. BA), subjects within the sequence, treatment (TRT vs. CON), and time periods on 2-h postprandial glucose values and area under the curve (AUC) after adjustment for covariates (i.e., age, sex, and race.). General linear models were used to test mean differences for 2-h postprandial glucose and insulin AUC values between two treatment groups across time periods after adjustment for covariates. All statistical procedures and analyses were conducted with SAS (version 9.4, PROC MIXED in SAS) software.
The baseline characteristics of study participants were expressed as mean ± SD and summarized in Table 2 (mean scores for age, 28 ± 11 years; BMI, 34.5 ± 7.5 kg/m2; waist circumference, 112.6 ± 13.8 cm; and fasting glucose, 5.1 ± 0.3 mmol/L).
Table 2: Baseline characteristics of study participants. View Table 2
As shown in Figure 1, the 2-h postprandial glucose AUC was significantly lower in the RS TRT than did the CON (12.5 ± 1.6 mmol/L•h vs. 15.6 ± 3 mmol/L•h, P = 0.002). When compared the glucose levels between the two treatments at each time point, there were significant mean differences at 30 minutes (6.6 ± 0.8 vs. 8.4 ± 1.3 mmol/L, P = 0.001), 60 minutes (6.6 ± 1.1 vs. 8.5 ± 2.1 mmol/L, P = 0.003), 90 minutes (6.3 ± 1.1 vs. 8.1 ± 1.8 mmol/L, P = 0.003), with borderline significance at 120 minutes (6.0 ± 0.8 vs. 7.4 ± 2.1 mmol/L, P = 0.052), respectively. Based on the 2-h glucose (incremental AUC 2.5 ± 1.3 mmol/L•h vs. 5.3 ± 2.6 mmol/L•h), the GI of the RS-supplemented muffins was 48 (glucose = 100).
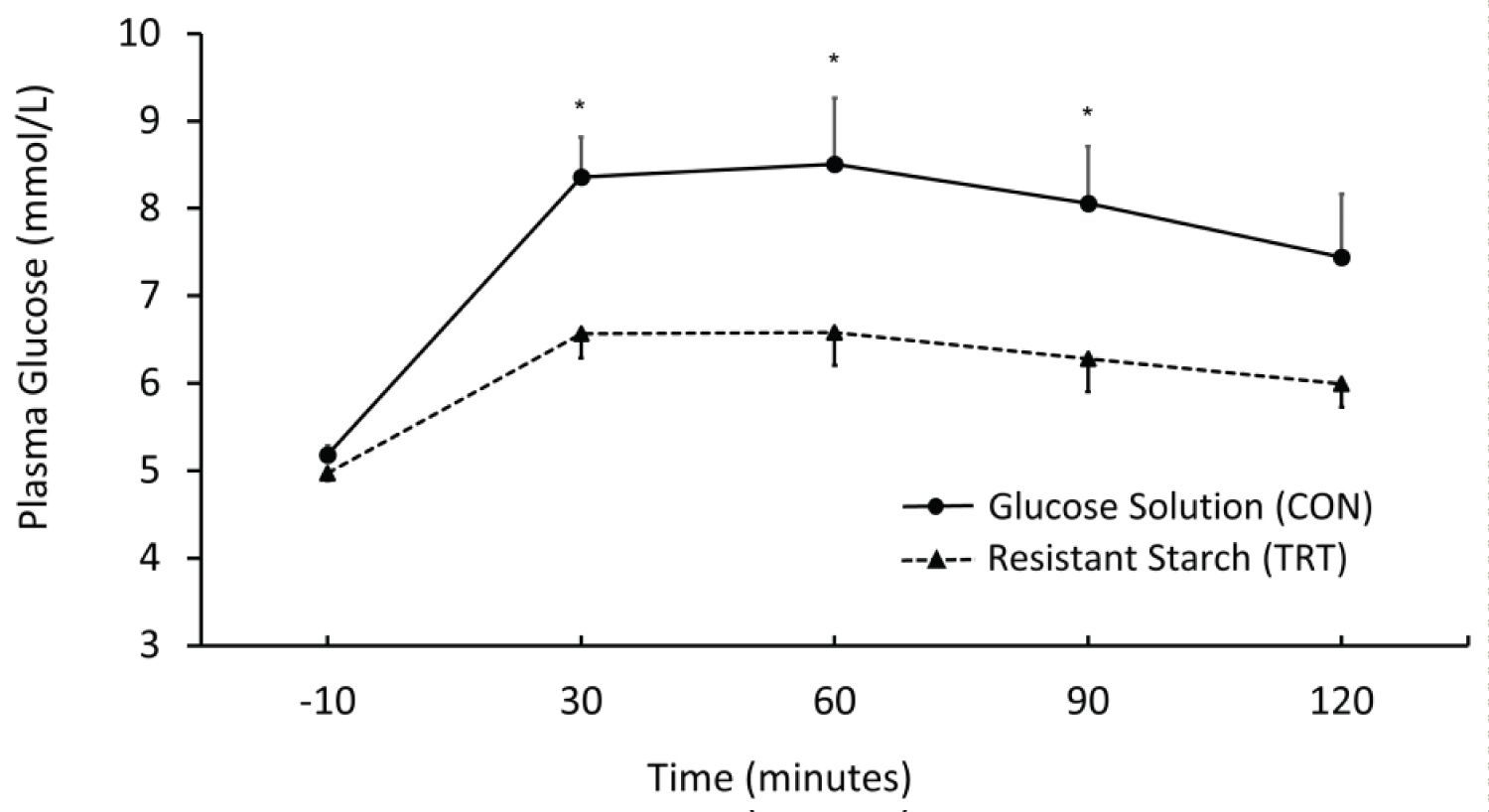 Figure 1: Postprandial glucose between TRT and CON conditions, *p < 0.05.
View Figure 1
Figure 1: Postprandial glucose between TRT and CON conditions, *p < 0.05.
View Figure 1
As shown in Figure 2, the 2-h postprandial insulin AUC was also significantly lower in the RS TRT than the CON (1354.5 ± 606 vs. 1788.9 ± 522.8 pmol/L•h, P < 0.001). When compared the insulin levels between the two treatments at each time point, there were significant mean differences at 60 minutes (806.9 ± 337.3 vs. 1090.2 ± 421.2 pmol/L, P = 0.02), 90 minutes (675.2 ± 338.4 vs. 1039.8 ± 227.8 pmol/L, P < 0.001), and 120 minutes (627.3 ± 277.7 vs. 934.3 ± 277.3 pmol/L, P = 0.046), except for 30 minutes (822.0 ± 406.2 vs. 892.6 ± 347.8 pmol/L, P = 0.4). In addition, all eight participants rate the palatability of the muffins above the "neither" level. Specifically, one rated the muffin as "like extremely," three rated as "like very much, " three rated as "like moderately," and one rated as "like slightly." The average rating was between "like very much" and "like moderately".
 Figure 2: Postprandial insulin difference between TRT and CON conditions, *p < 0.05.
View Figure 2
Figure 2: Postprandial insulin difference between TRT and CON conditions, *p < 0.05.
View Figure 2
Our major finding was that the RS-supplemented muffins induced significantly lower postprandial glucose and insulin levels than oral glucose solutions in sedentary and abdominally obese individuals. Based on the incremental glucose AUC values, the GI of the RS-supplemented muffins was 48. We also found out that the palatability of the tested muffins was satisfactory.
The RS applied in our study agreed with the US daily dietary fiber intake recommendations [22]. The US dietary guidelines recommend daily fiber intake ranging from 22 to 28 g in women and 28 to 33.6 g in men depending on the daily calorie needs [22]. This fiber intake level was also endorsed by the American Diabetes Association in their nutrition position statement for individuals with diabetes [25]. A meta-analysis of prospective studies revealed that individuals who consumed 27.9 g of total fiber per day had a 14% lower risk of developing type 2 diabetes than those who consumed 15.8 g of total fiber [26]. A randomized controlled trial in healthy persons showed that following a fiber-enriched diet (30g of total fiber per day) for three months lowered glucose levels by 12.3% compared with a control diet (10.4 g of total fiber per day). Our study potentially provides one promising way for the general public, especially individuals at risk of diabetes, to meet the recommended daily fiber intake. Our high dose of 30 g-RS is a reasonable daily fiber intake following recommendations by the American Heart Association (AHA) and the Institute of Medicine (IOM) [13,27]. The AHA recommended daily fiber intake of 25 to 30 g/day or 25 g or more per day [12,27], while the IOM recommended 21 to 25 g for women and 30 to 38 g for men [13].
Our findings regarding the blunted glucose responses to RS-supplemented muffins were consistent with previous studies [16,28-30]. Krezowski, et al. [28] examined the acute postprandial glucose responses to two types of corn starch muffins in patients with type 2 diabetes, which reported that the regular starch muffins had a GI of 103, while the RS muffins elicited a GI of 49, suggesting a glucose-lowering effect of RS supplementation. However, the exact amount of RS content per muffin was not reported in Krezowski, et al.'s study. Behall, et al. [16] also showed the attenuated glucose responses to RS-supplemented muffins among healthy, non-obese participants; the average RS content was 6.5 g, and the average total carbohydrates content was 72 g in each muffin meal with the GI of the muffin as 67. Our study with the RS-supplemented muffin had a GI of 48. We speculate that our study's lower GI observed was due to the higher RS dose in the tested muffins. Our study added 30 g of RS in each muffin that contained 75 g of digestible carbohydrates. In contrast, the average RS dose in Behall's study [16] was only 6.5 g per muffin with a similar amount of digestible carbohydrates. This difference implies that the high dose of RS in our study tended to produce additional glucose-lowering benefits compared to the lower RS doses in previous studies.
Studies that added RS to other types of foods also reported consistent findings. Li, et al. [19] demonstrated that the RS-enriched rice (8 g of RS per serving) reduced postprandial glucose responses by 37% compared with regular rice and glucose solutions in young, healthy individuals. The GI of RS-enriched rice was 48.4, while the GI of regular rice was 77.4 (glucose = 100) [19]. Similarly, Yamada and colleagues [31] examined the acute effect of adding 6 g of RS into 140 grams of bread on postprandial insulin and glucose responses than placebo bread. They showed that postprandial glucose levels were significantly lower at 90 minutes in the RS group than in the placebo group. Collectively, RS seems to have a wide application in modifying the glycemic responses to common carbohydrate-rich food.
The postprandial insulin levels in our study were lower in the RS TRT than did the CON. A previous study with young and healthy participants showed that adding 16.5 g of RS into white wheat bread containing 100 g of carbohydrates decreased the postprandial insulin rises by 43% (P < 0.05), as compared with white wheat control bread containing an equal amount of carbohydrates [32]. Similar findings have also been reported in individuals with abnormal fasting glucose [31].
There are several plausible mechanisms underpinning the effects of RS on GI and insulin responses. Robertson, et al. [33] suggest that RS, through fermentation in the gut microbiome, produces a series of metabolites, particularly short-chain fatty acids. The short-chain fatty acids were absorbed into the circulation and then inhibited the release of free fatty acids [34,35]. As shown in many studies, reduced levels of free fatty acids can improve insulin sensitivity [36,37]. However, another study demonstrated no significant fermentation occurred in the first 4 hours after the acute intake of RS by showing unchanged breath H2 levels, an indicator of colonic fermentation [19]. They also showed that the maximal fermentation started at 6 hours and was maintained for more than 10 hours [19]. Therefore, the acute decreased postprandial 2-hour glucose in our study was not likely caused by the colonic fermentation. We speculated that the blunted glucose and insulin responses were due to the delayed absorption of carbohydrates. Several in vitro studies have shown that high RS content slowed hydrolysis and increased carbohydrates viscosity, decreasing gastric emptying rate [38,39]. Direct evidence is still lacking in human individuals, and further studies are needed to investigate the mechanisms of the acute effect of RS on postprandial glucose and insulin responses.
Our study's interesting finding is that all eight participants rate their palatability of the muffins above the "neither" level. The most prevalent score was "like very much" and "like moderately". These findings indicated that adding RS to muffins was a suitable method of preparing fiber-rich, palatable starch foods. Our findings were also consistent with Sanz and colleagues [17], which compared the appearance, texture, and taste between the RS-containing muffins (10 g of RS per 100 g of muffins) and observed no difference between the two types of muffins. This is important because palatability has been shown as a decisive determinant of food choice and eating behavior [40].
A strength of our study is that we used an equal amount of digestible carbohydrates in intervention and control conditions. Given that the oral glucose solution provides 75 g of carbohydrates, we modified the muffin recipe so that the total carbohydrates in the RS-supplemented muffins were 105 g per serving, providing 75 g of digestible carbohydrates plus 30 g of RS. This approach was different from previous studies in which a certain portion of regular starch was replaced with RS, and the number of digestible carbohydrates was reduced [16,28-30]. Since the reduced amount of digestible carbohydrates can lead to lower postprandial glucose levels, our study provided more convincing evidence that the intake of RS supplementation rather than the reduced carbohydrates content attenuated the postprandial glucose levels following the consumption of starch-containing food. A limitation of our study is that we only measured glucose levels for 2 hours during the postprandial state. However, one previous study has shown that the ingestion of RS during breakfast improved glucose tolerance at lunch, suggesting a second meal effect [18]. Since the fermentation process may last up to 16 hours after an acute RS intake, it remains elucidated whether RS can impact glucose levels in the following meals after the second meal.
In conclusion, the RS muffins supplemented with 30 g of RS could reduce postprandial glucose and insulin responses in abdominally obese adults, meeting the USDA, AHA, and IOM daily fiber intake recommendations. The RS-supplemented muffin can be a promising food supplement for dietary fiber source with a low GI and acceptable palatability.
This work was supported by the Graduate and Professional Student Association at Arizona State University.
The authors declare no conflict of interests.