The flavonoids extracted from dried roots of Scutellaria species have been used in traditional Eastern medicine for the treatment of several human ailments, including cancer and inflammation. Modern science proved that wogonin is one of the major bioactive agents responsible for the physiological activity of Baikal skullcap (Scutellaria baicalensis Georgi.), which has been regarded as a potent anticancer agent. In this mini review, anti-inflammatory, antioxidant and anticancer activity of wogonin are discussed. Besides, the bioavailability of wogonin and utilization of nanotechnology to improve the bioavailability of wogonin are also presented.
Wogonin, Anti-inflammatory, Antioxidant, Anticancer, Scutellaria baicalensis
MNPs: Magnetic Nanoparticles; TPA: 12-O-Tetradecanoylphorbol-13-Acetate; NO: Nitric Oxide; ERK: Src-Extracellular-Signal-Regulated Kinase; NF-κb: Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells; Coxs: Cyclooxygenases; AA: Arachidonic Acid; IL-1b: Interleukin 1 Beta; TNF-α: Tumor Necrosis Factor-α; ICAM-1: Intercellular Adhesion Molecule 1; Inos: Inducible Nitric Oxide Synthase; Myd88: Myeloid Differentiation Primary Response 88; TAK1: Tgfbeta-Activated Kinase1; Mapks: P38 Mitogen-Activated Protein Kinases; LPS: Lipopolysaccharide; DRG: Dorsal Root Ganglion; HTSE: Hamster Tracheal Surface Epithelial; TRAIL: Tumor Necrosis Factor Related Apoptosis Inducing Ligand; HTLV1: Human T-Cell Leukemia Virus Type 1; ATL: Associated Adult T-Cell Leukemia/Lymphoma; C-FLIP: Cellular FLICE (FADD-Like IL-1β-Converting Enzyme)-Inhibitory Protein; ALI: Acute Lung Injury; BALF: Bronchoalveolar Lavage Fluid; PPAR-Γ: Peroxisome Proliferator-Activated Receptors; STAT1/3: Signal Transduction And Transcription 1 And 3; P-Akt: Serine-Threonine Kinase; Phosphoinositide-3-Kinase (PI3K)/Akt; CD: Cyclodextrin; HP-B-CD: Hydroxypropyl-Cyclodextrin; STZ: Streptozotocin; MDA: Malondialdehyde; T-GSH: Total Glutathione; NP-SH: Non-Protein Sulfhydryl; SOD: Superoxide Dismutase; CAT: Catalase; ER: Endoplasmic Reticulum; SCI: Spinal Cord Injury; Bcl-2: B-Cell Lymphoma 2; Bax: BCL2 Associated X; ROS: Reactive Oxygen Species; XIAP: X-Linked Inhibitor Of Apoptosis Protein; Ciap: Cellular Inhibitor Of Apoptosis Protein; C-Myc/SKP2/Fbw7a: Cellular-Myc/S Phase Kinase Associated Protein 2/F Box And WD Repeat Containing Protein 7; HDAC1/HDAC2: Histone Deacetylase1/2; U-2 OS: Osteosarcoma Cell Line; P-Gp: P-Glycoprotein; Htert: Human Telomerase Reverse Transcriptase; Htp1: Human Telomerase-Associated Protein 1; CDK9: Cyclin-Dependent Kinase 9; NPC: Nasopharyngeal Carcinoma; NAC: N-Acetyl Cysteine; Pacs: Phyto-Active Chemicals; MDR: Multidrug Resistance Proteins; MTT: 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide; DAPI: 4′,6-Diamidino-2-Phenylindole; GA-WG-Lip: (GA)-Modified WG Liposome
Cancer or malignant neoplasm is a group of deadly diseases that results from genetic or epigenetic alterations in the somatic cells. The abnormal cells with rapid creation grow beyond their usual boundaries, can spread to the other organs and tissues [1]. Nowadays, several treatments are using against cancer including the surgery, chemotherapy, radiation therapy, targeted therapy and immunotherapy. However, these treatment methods might cause side effects and problems by affecting the healthy tissues or organs in patients. For instance, anemia, edema, fatigue, hair loss, nerve problems, pain are the most common side effects which arise by cancer treatments [2]. The side effects can be more important if the patient is old and frail [3]. Therefore, it is important to find alternative or complementary cancer treatments with fewer detrimental side effects and the low toxicity associated with the current chemotherapeutics. The anticancer properties of plants have been recognized for centuries. One of the most important group of plant secondary metabolites with anti-cancer activities, are flavonoids which have a common phenolic structure. The role of dietary flavonoids in cancer inhibition demonstrate that flavonoids are chemo preventive or therapeutic agents against cancer [4]. Flavonoids also have great antioxidant, antimutagenic, antibacterial and anti-inflammatory activities [5]. Flavonoids are phytochemical components that could find as free aglycones or glycoconjugates. Wogonin (5,7-dihydroxy-8-methoxyflavone) is an active component originated from the roots of S. baicalensis. Wogonin (Figure 1A) as a glycoconjugate, wogonoside, (Figure 1B) was found in 4 species of the Scutellaria genus [6].
 Figure 1: Chemical structure of (A) aglycone wogonin and (B) wogonoside. View Figure 1
Figure 1: Chemical structure of (A) aglycone wogonin and (B) wogonoside. View Figure 1
The plants which are belong to the Scutellaria genus constitutes one of the common components of Eastern as well as traditional American medicine which widely have been used for clinical treatment of hyperlipidemia, atherosclerosis, hypertension, dysentery, common cold, viral, bacterial, inflammatory disease and cancer [7]. Plants of the genus Scutellaria (Lamiaceae family) is widely distributed throughout the world and is represented by close to 350-400 species [8]. The Chinese herb S. baicalensis is one of the most common plants in the genus Scutellaria which is widely used as a traditional treatment of cancer due to the wogonin contain (Figure 2). Wogonin displays a great variety of pharmacological activities including antioxidant [9], anti-inflammatory [10], anxiolytic [11], anti-hepatitis [12], anticonvulsant [13], anti-angiogenesis [14], neuroprotective [15,16] and anticancer [17-19]. Due to their remarkable spectrum of biological activities, flavonoids drew greater attention among the various natural products [5].
 Figure 2: Scutellaria baicalensis (Baikal skullcap). View Figure 2
Figure 2: Scutellaria baicalensis (Baikal skullcap). View Figure 2
Despite the wogonin displays a great variety of pharmacological activities the use of wogonin is limited by poor solubility and low bioavailability like other flavonoids [20,21]. However, with the increasing rate of cancer in the world, the new strategies are being explored to improve the bioavailability, therapeutic activity and selectivity of wogonin and other anti-cancer agents [21]. To solve the solubility and targeting problems, liposomes [22-24], magnetic nanoparticles (MNPs) [25,26] and salification technology [20] can be used as drug delivery system. In this mini review, anti-inflammatory, antioxidant and anticancer activity of a specific flavonoid, wogonin which found in Scutellaria genus will be discussed. Moreover, the bioavailability of wogonin and utilization of nanotechnology to improve the bioavailability of wogonin is also presented.
The anti-inflammatory properties of wogonin, such as inhibition of nitric oxide (NO) [27], 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced cyclooxygenase-2 expression [10,28] and prostaglandin E2 production [29-32] have been reported previously. Yeh, et al. [33], revealed that the Endotoxin-Induced Prostaglandin E2 and Nitric Oxide production can be attenuated via Src-extracellular-signal-regulated kinase (ERK)1/2- nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway in BV-2 microglial cells by wogonin. Flavonoids especially wogonin can inhibits the activity of inflammation-associated enzymes, such as cyclooxygenases (COXs) and lipoxygenases [32,34]. Wogonin plays role as an expression regulatory of inflammation-associated proteins [32,34]. Wogonin had been referred as a selective COX-2 inhibitor [35,36]. The effects of wogonin on the expression of COX-2, Interleukin 1 beta (IL-1b) (treated by arachidonic acid (AA)) tumor necrosis factor-α (TNF-a), and Intercellular Adhesion Molecule 1 (ICAM-1) (treated by TPA) have been reported by Chi, et al. [37]. In TPA-induced inflammation, wogonin potently reduced COX-2 and TNF-a gene expression while ICAM-1 and IL-1b were weakly affected. The same results were observed in an acute-type model of AA-induced inflammation. These results show that wogonin can differentially regulates the expression of inflammation-associated genes in vivo [37]. The suppression activity of wogonin on the cyclooxygenase-2 expression in skin fibroblasts also has been reported previously [38]. Wogonin treatment against the contact dermatitis in the animal models, showed that wogonin inhibits the expression of IL-1b, COX2, interferon- γ, intercellular adhesion molecule-1 and inducible nitric oxide synthase (iNOS) [39]. The inflammatory effect of wogonin on the gene expression of cyclooxygenase-2 in human lung epithelial cancer cells also reported by Chen, et al. [40]. Their results showed that wogonin suppressed the COX-2 expression and also has the neuroprotective effects [40]. The inhibitor effects of wogonin on the gene expression of TNF-α, IL-1b and iNOS in cultured brain microglia have been reported by Lee, et al. [15]. The reduction in the expression of inducible NO synthase and NF-κB activation results to NO production suppression [15]. In addition, it has been demonstrated that wogonin inhibits the expression and interaction of Toll-like receptor 4 (TLR4), Myeloid differentiation primary response 88 (MyD88) and TGF beta-activated kinase1 (TAK1). Wogonin can decreases the activation of NF-κB and p38 mitogen-activated protein kinases (MAPKs) pathway in lipopolysaccharide (LPS)-Induced Dorsal Root Ganglion (DRG) neurons [36]. Wogonin suppresses lipopolysaccharide and lipoteichoic acid-induced iNOS gene expression and NO production while the Nor-wogonin (a structural analogue of wogonin with an OH substitution at C8) has not the same effect [41]. Roots of the S. baicalensis, has been used for the treatment of various chronic inflammatory diseases such as respiratory disease in oriental medicine [42]. Heo, et al. [43] reported that treatment of the hamster tracheal surface epithelial (HTSE) cells with wogonin, significantly inhibited ATP-induced mucin release by directly acting on airway mucin-secreting cells. The authors suggested that wogonin can be used as mucoregulators during the treatment of chronic airway diseases. Tang, et al. [44] investigated the effects of wogonin on the expression of interleukin-1α (IL-1α), TNF-α and NF-κB in leukocytes from Sprague-Dawley rats. The results showed that wogonin increased the IL-1α and TNF-α mRNA and can also over express these genes. These results showed the possible effects of wogonin on the IL-1α and TNF-α mRNA expression by affecting the NF-κB in rat leukocytes.
TNF-α is a potential anti-cancer agent, however the most of the cancer cells are resistant to TNF-induced cell death, it might cause by the activation of NF-κB. Therefore, the sensitizing of the cancer cells to TNF-induced cytotoxicity by blocking of NF-κB can help the cancer cells death [45]. Wogonin synergistically sensitizes cancer cells derived from the cervix, ovary and lung to TNF-induced apoptosis by inhibition of catalase activity [46]. In addition, wogonin-induced reactive oxygen species block TNF-induced NF-κB activation [46].
Tumor necrosis factor related apoptosis inducing ligand (TRAIL) is a potential anticancer agent that kills tumor cells without any side effects on normal tissues [47]. However, most cancer cells remain refractory to TRAIL. Thus, developing combination therapies using sensitizers of the TRAIL pathway is one of the efficacious approaches. Ding, et al. [48] used TRAIL resistant human T-cell leukemia virus type 1 (HTLV1) associated adult T-cell leukemia/lymphoma (ATL) cells as a model system for investigation the potential sensitizers of TRAIL induced apoptosis. The results showed that wogonin and the flavones apigenin and chrysin breaks TRAIL resistance in HTLV1 associated ATL by inhibition of Cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein (c-FLIP) transcription and by upregulation of TRAIL receptor 2 (TRAILR2). cFLIP is a antiapoptotic factor and key inhibitor of death receptor signaling. Yang, et al. [46] have shown that wogonin inhibited the expression of the c-FLIP, which is coupled with potentiation of TNF-induced caspase 8 activation that initiates apoptosis. Wogonin inhibits the TNF-a production in LPS stimulated RAW cells in vitro and reduces the level of circulating TNF-α in mice administrated D-galactosamine and LPS in vivo [49]. The ERK, MAPK, and c-Jun N-terminal kinase (JNK) proteins in MAPK pathway, regulate various cellular functions of mammal leukocytes, such as activation, chemotaxis, and proliferation [33,50,51]. They are important upstream regulators for the induced expression of proinflammatory mediators NO and cytokines in LPS-induced acute lung injury (ALI) [50,52,53]. Wei, et al. [54] investigated the inhibitory effect of wogonin on the phosphorylation of ERK, p38 MAPK, and JNK in LPS-induced ALI in vivo. The authors found that pre-administration of wogonin inhibited lung edema, protein accumulation in bronchoalveolar lavage fluid (BALF), expression of iNOS and COX-2, phosphorylation of p38 MAPK and JNK of LPS-induced ALI. These results showed that wogonin has predominantly protective effect against LPS-induced ALI by inhibition expression of iNOS and COX-2 by blocking phosphorylation of p38 MAPK and JNK proteins in MAPK pathway. Furthermore, recent study by Yao, et al. [55] demonstrated that LPS-induced ALI in female C57BL/6 mice is prevented by wogonin. Lee & Park [56], demonstrated the effects of wogonin on virus-induced macrophages. The represented results showed that wogonin has anti-inflammatory properties due to its inhibition of nitric oxide, cytokines, chemokines, and growth factors in double strand RNA-induced macrophages via the calcium-STAT pathway.
The collaboration of wogonin with DNA at preventing the denaturation of DNA strands and providing of stability to genomic DNA against variety of chemical denaturants have been shown by Khan, et al. [57] (Figure 3). Recently, Li, et al. [58] showed that wogonin acts as one of the effective agonist of peroxisome proliferator-activated receptors (PPAR-γ) in alcoholic liver disease. Thus, wogonin might acts as an efficient modulator of PPAR-γ expression activity. Some studies revealed that blocking phosphorylation of signal transduction and transcription 1 and 3 (STAT1/3), Janus kinases and p38 MAPK by wogonin can downregulate the expression of iNOS and COX-2 and attenuate the production of IL-6 and TNF-a [54,59] (Figure 3). TRAIL is a promising therapeutic agent against different types of cancer that kills various tumor cells without damaging normal tissues. However, many tumors remain resistant to TRAIL. A combination therapies using sensitizers of the TRAIL pathway is an effective approach to dominate TRAIL resistance. Wogonin is one of these therapies that can break TRAIL resistance. Ding, et al. [48] showed that wogonin can break the TRAIL resistance by transcriptional downregulation of cFLIP (inhibitor of death receptor signaling) and by upregulation of TRAILR2.
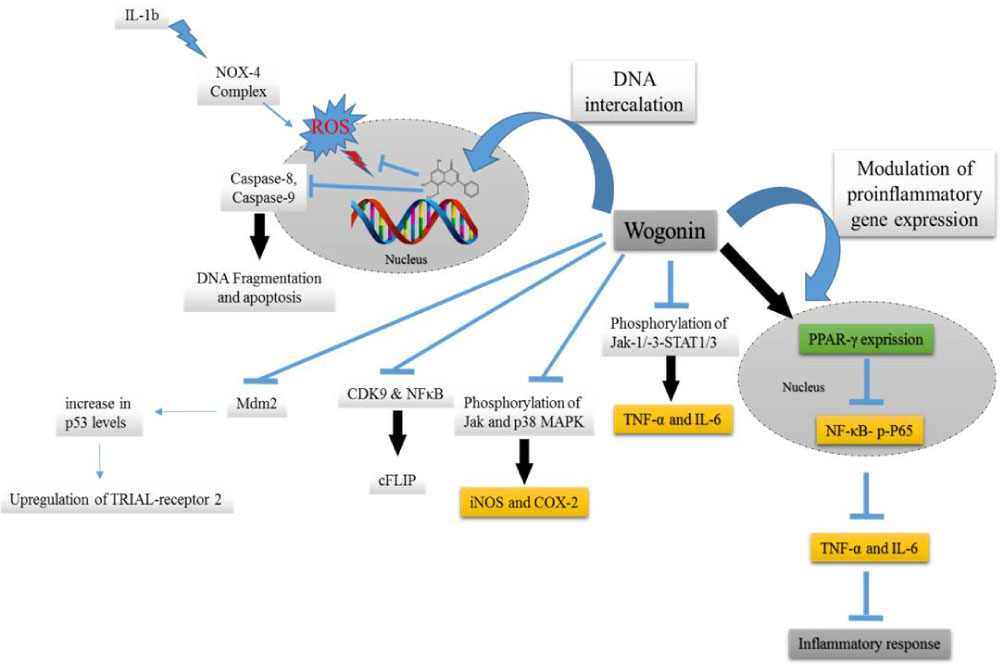 Figure 3: Anti-inflammatory properties of wogonin via several signaling pathways. View Figure 3
Figure 3: Anti-inflammatory properties of wogonin via several signaling pathways. View Figure 3
The anti-oxidant activity of wogonin has been reported previously [60]. Recent study showed that wogonin inhibits H2O2-induced vascular permeability by downregulating the phosphorylation of caveolin-1 associating with the suppression of stabilization of VE-cadherin and -catenin in human umbilical vein endothelial cells (HUVECs) [60]. Wang, et al. [61] found that wogonin can suppress the H2O2-stimulated action remodeling and albumin uptake of HUVECs, as well as transendothelial cell migration of the human breast carcinoma cell MDA-MB-231. It has been reported that the supplementation of wogonin could reduce the hydroperoxide-induced apoptosis by a significant upregulation of a serine-threonine kinase (p-Akt) expression in human APRE cell lines [62]. It could be deduced that the protective effect might be conducted via phosphoinositide-3-kinase (PI3K)/Akt pathway [62]. Excitotoxicity is the pathological process by which neurons are damaged and killed by the over activation of receptors for the excitatory neurotransmitter glutamate, such as the NMDA and AMPA receptors [63]. Cho & Lee [63] evaluated the effects of wogonin on excitotoxicity as well as various types of oxidative stress induced neuronal damage in primary cultured rat cortical cells. Their results showed that wogonin exhibits neuroprotective actions in cultured cortical cells by inhibiting excitotoxicity. Wogonin can also inhibit several types of oxidative stress-induced neuronal damage. Its antioxidant effects with radical scavenging activity may contribute to the neuroprotective effects. Free radical scavenging activity of phenolic compounds is related to the number and structure of phenolic hydrogen in their molecules [9]. Thus, even only a very small structural difference in the flavonoid skeleton would lead to a marked difference in the potency of the antioxidative and anti-inflammatory activities [64]. Huang, et al. [64] studied the antioxidative and anti-inflammatory effects of the flavones: Baicalein, oroxylin A and wogonin. They showed that all these flavones exhibit significant antioxidative and free-radical scavenging activities. Among them wogonin displayed extremely potent inhibition of NO production and strong antioxidative activity. These results suggested that the potent activity of wogonin was related to its unique structure [63]. To improve the solubility, chemical stability and bioavailability of poorly soluble compounds, cyclodextrin (CD) complex can be used effectively [65]. In the recent years some studies had been reported the coverage of wogonin with CDs, including b-CD and hydroxypropyl-cyclodextrin (HP-b-CD) in solution [58,66]. Li, et al. [66] synthesized the complexes of wogonin with b-CD and HP-b-CD by refluxing method, and determined the effect of the complexation process on their antioxidant capacity by DDPH assay. The experimental results showed that the Wogonin and CDs complexes had anti-oxidant activity and wogonin/HPb-CD complex was the most reactive form. In the inhibition of the hydroxyl radical-induced peroxidation of linoleic acid assay, wogonin plays a role as a protection of linoleic acid against oxidation [67].
Wogonin also has a remarkable role in reducing liver damage [68]. It caused by streptozotocin (STZ)-induced diabetes in rats due to alleviating of malondialdehyde (MDA), as a lipid peroxidation product. Wogonin also can elevate the total glutathione (T-GSH) because of its antioxidant, non-protein sulfhydryl (NP-SH), superoxide dismutase (SOD) and catalase (CAT) activity [68].
The critical roles of endoplasmic reticulum (ER) stress in a range of neurological disorders such as Spinal cord injury (SCI) had been shown recently [69-71]. Remarkably, wogonin has widely clinical efficiency in several nerve system diseases [15]. The anti-oxidant and anti-inflammatory properties of wogonin is the main feature of its neuroprotective effect [71]. Xu, et al. [71] showed that wogonin can protect DRG neurons death by preventing tunicamycin-induced ER stress in vitro.
Wogonin has anticancer activity against several of cancers (Table 1). The inhibitory effect of wogonin was investigated in HT-29 human colorectal cancer cells [72]. Treatment with wogonin decreased the expression of B-cell lymphoma 2 (Bcl-2) and increased the expression of BCL2 Associated X (Bax) was in a dose-dependent manner compared with the control. Furthermore, the induction of apoptosis was coupled with an inactivation of PI3K/Akt in a dose-dependent manner [72]. It has been reported that wogonin induced prolonged elevation of intracellular Ca2+ levels in malignant T cells, leading to reactive oxygen species (ROS) accumulation and eventual apoptosis [18]. Wogonin has been proven to enhance antitumor activity of TRAIL in vivo [73]. The co-treatment with wogonin and TRAIL of non-small-cell lung cancer xenografted tumor model in nude mice showed that, the expression levels of antiapoptotic proteins, including long form of cFLIPL, X-linked inhibitor of apoptosis protein (XIAP), and cellular inhibitor of apoptosis protein 1 and 2 (cIAP-1 and cIAP-2) were markedly reduced [73]. It indicated the wogonin triggered apoptosis via ROS-mediated downregulation of cFLIPL and IAP proteins [73]. In wogonin-treated murine sarcoma S180 cells, the bax and p53mRNAs level increased and the level of bcl-2 mRNAs decreased. It shows pro-apoptotic effect of the wogonin through the inhibiting tumor growth activity both in vitro and in vivo [17]. The inhibitory effect of wogonin was investigated in human lung adenocarcinoma cell line A549 by Regulating c-Myc/S phase kinase associated protein 2/Fbox and WD repeat containing protein 7 (c-Myc/SKP2/Fbw7a) and histone deacetylase1/2 (HDAC1/HDAC2) Pathways [74]. Parajuli, et al. [75] reported that the leaf extract (SocL extract) of Scutellaria Ocmulgee Small, which showed some of the best anti-proliferative activity, contained only wogonin. These results suggested that probably wogonin could potentially have very high anticancer activity among the flavonoids examined and could have positive interaction with other phytochemicals even at low concentration. The investigation of wogonin effects on human osteosarcoma cell line (U-2 OS) showed that a dose- and time-dependent reduction occurred in cell viability after exposure to wogonin [76]. Wogonin triggers apoptosis in U-2 OS cells through the activation of caspase-3, induction the production of ROS and intracellular Ca2+, and altered the levels of apoptotic proteins [76] or ribonucleic acid (m-RNA) expression in the HL-60 leukemia cells [77]. Tumor initiation, progression and resistance to conventional chemotherapies are tightly associated with over-expression of anti-apoptotic Bcl-2 family proteins Bcl-2, Bcl-xL, Bcl-w and Mc. Enomote, et al. [78] demonstrated that wogonin is likely to potentiate the antitumor activity of etoposide and ameliorates its adverse effects through potentiating of anticancer action of etoposide. This effect is seems to be due to P-glycoprotein (P-gp) inhibition activity of etoposide and accumulation of P-gp. Wogonin has been reported to induce apoptosis through down-regulation of Bcl-2 and inhibits of human telomerase reverse transcriptase (hTERT), human telomerase-associated protein 1 (hTP1) and c-myc messengl-1 (myeloid cell leukemia) [79]. Navitoclax (ABT-263), an orally bio-available small-molecule mimetic of the Bcl-2 homology domain 3, which inhibit Bcl-2, Bcl-xL, and Bcl-w with high affinities [80]. ABT-263 has shown anti-cancer effects mainly on hematological malignancies [81,82]. Polier, et al. [83] showed that the anti-cancer flavone, e.g., wogonin targeting cyclin-dependent kinase 9 (CDK9) and potentiates the anti-cancer efficacy of the Bcl-2 family inhibitor ABT-263. They showed that wogonin enhances ABT-263-induced apoptosis in different cancer cell lines. In combination with ABT-263 wogonin, promotes in vivo tumor regression in a human T-cell leukemia xenograft mouse model. It indicated that wogonin reduce the effective dose of ABT-263 thereby possibly decreasing the risk of adverse side effects. A recent study by Dandawate, et al. [84] showed that SocL and flavonoid wogonin could inhibit TGF-b1-induced Treg activity in malignant gliomas. Their studies revealed that inhibition of TGF-b1-induced Treg activity by SocL or wogonin may involve to a certain extent, an inhibition of ERK 1/2 (P44/42) MAPK activity [84].
Table 1: Anticancer activity of wogonin against various cancers. View Table 1
It is well-appointed that anticancer agents induce apoptosis, which is necessary to keep cellular homeostasis and normal development. Chow, et al. [85] carried out the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazole (MTS), flow cytometry, and Poly (ADP-ribose) polymerase (PARP) cleavage assays to assess antitumor activity and the mechanistic action of wogonin in human nasopharyngeal carcinoma (NPC) cells. The results revealed that wogonin induced apoptosis in human NPC cells by inactivation of GSK-3b via prominent inhibition of phosphorylation at Tyr216 and slightly increment of phosphorylation at Ser9. Furthermore, wogonin was shown to induce dose-dependent cell apoptosis due to the induction of sub-G1-phase cells, PARP cleavage, and downregulation of DNp63 [85]. Yu & Kim [86] has found that wogonin induces the mitochondria and death-receptor-mediated apoptotic cell death by activation of several caspases, induction of PARP cleavage, changing the antiapoptotic/proapoptotic Bcl-2 family member ratios and cleavage of Bid. Further investigation by them revealed that generation of ROS was an important mediator in wogonin-induced apoptosis. Wogonin induces apoptosis by activation of ERK and p38 MAPKs signaling pathways by inhibition of N-acetyl cysteine (NAC), a ROS scavenger, and generation of ROS in human MCF-7 breast cancer cells. It indicated that wogonin-induced ROS are associated with MAPKs activation of ERK and p38 MAPKs signaling pathways [86]. Chen, et al. [86,87] carried out MTS to assess the antiproliferative activity of the Phyto-active chemicals (PACs) oridonin and wogonin in chemo-resistant epithelial ovarian cancer cells. The result of MTS assay revealed that oridonin and wogonin may have combined activity in ovarian cancer in advanced-stage, following its development of resistance to carboplatin and paclitaxel (Table 1).
It’s very important to investigate the pharmacological effects of drugs on animal models in vivo. Previous pharmacokinetic studies showed that wogonin has low oral bioavailability as one of the active agent of traditional remedies [11,21], however, the wogonoside, glycosylated product of wogonin, has high plasma concentration and bioavailability after oral administration [88]. The observed low oral bioavailability of wogonin could be resulting of the compound low water solubility [20]. MNPs and liposomes are important useful methods for increasing of solubility and drug delivery of pharmaceutical drugs. MNPs can be used as a diagnostic and cancer treatment tool due to its large surface-to-volume ratio, low toxicity, biodegradable nature, super paramagnetic property and biocompatibility properties [89,90]. The application of drug-coated MNPs can be used to increase solubility of the drugs and to enhance its chemotherapeutic efficiency as an important tool for cancer treatment [25,91]. For instance, Cheng, et al. [24] used the magnetic nanoparticles with wogonin and Fe3O4, as a system for targeting the drug delivery, to study the reversal of multidrug resistance proteins (MDR) by downregulating MDR1 in K562 cells. The results showed that it cans significantly downregulate the transcription of MDR1 mRNA and expression of P-glycoprotein in K562/A02 cells. Additionally, the reversible effect, apoptosis rate and accumulation of intracellular daunorubicin of daunorubicin-wogonin magnetic nanoparticles were significantly higher than daunorubicin + wogonin and daunorubicin magnetic nanoparticle groups. These findings suggested that the magnetic nanoparticle formulation of wogonin would be a promising strategy for overcoming multidrug resistance in cancer cell [25].
Due to nontoxic, biocompatible, and biodegradable property, liposomes can be used as a versatile and effective nanometer-scale drug delivery system [92,93]. It has been demonstrated that liposomes can encapsulate both lipophilic and hydrophilic as well as amphoteric drugs [94,95]. Liposomes also can improve the oral bioavailability of poorly bioavailable drugs by increasing drug solubility and stability [96,97]. Liposomes have been applied to carry anticancer drugs with strong toxic effects for live tumor therapy. Liposomes also are very useful in targeted therapy of liver cancer [98]. They incorporated of wogonin in a novel glycyrrhetinic acid (GA)-modified WG liposome (GA-WG-Lip) that can rapidly accumulate in the liver with a long retention time, and has a better tumor inhibitory ratio than that of the unmodified liposomes due to increased receptor-mediated uptake of liposomes by liver-targeted cells [98]. Wogonin in the solution formulation seems to be better dissolved in the intestinal juice and well transported across the intestinal epithelial cells. Because wogonin does not produce significant cytotoxicity, increase apoptosis in multidrug-resistant cancer cells, or decrease induction of apoptosis in normal cells [99] it could be used an ideal P-gp inhibitor with high efficiency and low toxicity [100].
This work was supported by School of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran.
The authors declare that they have no conflict of interest.