Compared to other livestock, domestic fowls are much more commonly reared and consumed in Nigeria. The emergence of serious live-threatening infections from veterinary sources and treatment failures occurring with the available antibiotics warrants investigation into the use of antimicrobial agents in poultry farms and how they contribute to the menace of antibiotic resistance. The main aim of this study was to investigate the use of antimicrobial agents in poultry farms by poultry farmers in Ile-Ife and the prevalence of resistance in bacterial isolates from the poultry. The study was carried out in two stages which comprised of field work and laboratory investigations.
A total of 60 questionnaires were distributed to farmers patronizing two well known poultry drug seller shops in Ile-Ife. Ten out of the 60 farms were subsequently visited for sample collection. Fresh feacal samples were collected from the farms for isolation and identification of E. coli and Staphylococci which were subsequently subjected to antimicrobial susceptibility tests.
All the poultry farmers used one or more antibiotics for their birds. Antibiotics were used mostly for therapeutic and prophylactic purposes and to a lesser extent for growth promotion. Cotrimoxazole and neomycin were the most commonly used antibiotics. The E. coli strains were more resistant than the staphylococci and showed 100% resistance to nalidixic acid and amoxicillin but with lower resistance to gentamicin.
Our findings provide additional evidence that the poultry production environment in Nigeria represents an important reservoir of antibiotic resistance genes which poses potential public health risk to human populations.
Compared to other livestock, domestic fowls are much more commonly reared in Nigeria [1,2]. The reasons for this are not far-fetching. It has been shown that the domestic fowl grow fast and have high financial returns with few social, health and religious taboos against its consumption, usage and production in contrast to other livestock [2,3]. Furthermore, in Nigeria, poultry meat and eggs are among the main source of protein in most homes [1].
Most commercial farmers rearing chicken for meat or layers for eggs rear them under intensive management system [4]. The farmers do everything possible to care for their fowls in order to prevent diseases and death and thus increase profitability. Apart from feeding, the next instrument in the arsenals of farmers in order to ensure this is the use of veterinary drugs. Antimicrobials comprised the major components of veterinary drugs [5]. These drugs are supplemented in poultry feeds at sub-therapeutic levels for growth improvement, prevention or reduction of disease outbreaks, improvement of digestion, acceleration of weight gain and increase in feed conversion ratio [5,6].
Despite the benefits derived from the use of antimicrobial agents in poultry and other livestock production, the increased use has been shown to contribute to the increasing prevalence of bacterial antibiotic resistance in humans [7-9]. Reports revealed widespread presence of antibiotic-resistant pathogens in poultry farms and products in many areas around the world [10-13]. Isolated pathogens include Staphylococcus aureus, Campylobacter, Salmonella, Escherichia coli, Proteus mirabilis, and some other enterobacterias [12-19].
E. coli isolated from Portuguese poultry were reported to have high resistant tetracycline (70%), ampicillin (63%) while low level of resistance was observed with co-trimoxazole (33%), gentamicin (17%) and co-amoxiclav (17%) [20]. Similarly, Salmonella isolated from retail raw poultry in China was resistant to sulfisoxazole (74.1%), tetracycline (71.1%) and to a lower extent, cefoxitin (19%). Only 4% of the isolates were sensitive to all the antibiotics tested [21]. In Jamaica, E. coli isolated from broiler chickens were resistant to kanamycin (91.2%), nalidixic acid (85.3%) and ampicillin (20.6%) but all the isolates were sensitive to gentamicin [22].
Antibiotic resistant organisms can get to the general population from the farm through food chain or animal handlers [17] and through the application of animal manure on crop lands [22-24]. Furthermore, it has been shown that farm-raised superbugs can exchange genetic materials and give their resistance to other bacteria, even of other genera and species that have never been exposed to antibiotics [18,25,26].
The emergence of serious live-threatening infections from veterinary sources and treatment failures occurring with the available antibiotics warrants investigation into the use of antimicrobial agents in poultry farms and how they contribute to the menace of antibiotic resistance [27-29]. Attempts to control the emergence of antibiotic resistance in humans will therefore involve taking care of its occurrence in animals, particularly poultry which are consumed by a large proportion of people in Nigeria.
The main aim of this study is to investigate the use of antimicrobial agents in poultry farms by poultry farmers in Ile-Ife and the prevalence of resistance in bacterial isolates from the poultry feaces.
Ethical approval was obtained from Osun State Health Research Ethics Committee, Osun State Ministry of Health (OSHREC/PRS/569T/123). The consents of selected farmers were sought and obtained after explaining the purpose of the study.
This study was carried out in Ile-Ife of Osun State, in South-West of Nigeria. Ile-Ife is a semi-urban town with two Local Government Areas, namely Ife Central and Ife East Local Government Areas. Geographically, Ile-Ife lies on longitude 4°69'E and latitude 70°50'N and has a humid tropical climate with distinct wet and dry seasons. Rainy season starts April through October while the dry season lasts October to March. Ile-Ife is an ancient town in Yoruba history and is regarded as the cradle of civilization [30]. Ile-Ife can be divided into five areas based on the major roads in the town: Ede road, Ibadan road, Ondo road, Sabo/ Ilesha road and road 7/campus.
This involved the use of questionnaires. A set questionnaire was designed to survey the antibiotics commonly used by farmers in their poultry farms. Two well known poultry drug seller shops in Ile-Ife were selected and visited twice in a week each for two consecutive weeks. Questionnaires were administered to clients (poultry farmers) that patronize these poultry drug sellers who gave informed consent to participate in the survey.
A total of 60 questionnaires were administered in the study period: 45 questionnaires were administered in the shop that had higher patronage and 15 questionnaires in the second. The questionnaires were grouped into five based on the five major roads in Ile-Ife town as already indicated.
Data gathered with the questionnaire were coded and entered into IBM/ SPSS version 21. This was used to generate frequencies and percentages.
Two filled questionnaires each were picked at random from each of the five groups making a total of ten questionnaires representing ten farms. The ten selected farms were visited for sample collection. Fresh fecal samples were collected in sterile universal bottles from the ten poultry farms. Samples were taken immediately to the laboratory for isolation and identification of E. coli and Staphylococci; and for antimicrobial susceptibility tests.
Feacal samples were plated on MacConkey Agar for E. coli and Mannitol Salt Agar for staphylococci isolation. Plates were incubated at 37 ℃ for 24 to 48 hours. Morphological characteristics of the colonies were noted for characterization. Further characterization of isolates involved biochemical tests such as catalase tests, indole production and sugar fermentations [10,13].
Few colonies from the plates were inoculated in 2 ml sterile distilled water, and shaken using a rotamixer for uniform dispersion until a turbidity conforming to 0.5 McFarmland Barium Sulphate standard unit (average turbidity, 108 cfu/ml) was obtained in accordance to standard guidelines [31]. Surface of the over-dried Mueller Hinton Agar plates were swabbed with the dispersion. Antibiotic discs were placed on the plates with the aid of flamed forceps and plates were then incubated in the refrigerator for an hour and subsequently at 37 ℃ for 24 hours. Antibiotics screened reflected both the ones being used by the poultry farmers as well as the commonly available. The antibiotics screened include: Augmentin® (30 µg), gentamicin (10 µg), nalidixic acid (30 µg), chloramphenicol (30 µg), cloxacillin (5 µg), erythromycin (5 µg), penicillin (1i.u.), streptomycin (10 µg), tetracycline (25 µg), ampicillin (10 µg), amoxicillin (25 µg) and cotrimoxazole (25 µg) (Abtek, England). The diameter of the zones of inhibition was measured for each of the disc and used for interpretation following standard guidelines [31].
Data gather with the questionnaires were coded and entered into IBM/SPSS version 21. This was used to generate frequencies and percentages. Spearman's coefficient was used to determine correlation of parameters obtained from questionnaires and laboratory experiments.
As shown in Table 1, majority of the poultry farmers were male (81.9%) and possessed a University degree (51.7%). Most (66.7%) of them however, had other occupations apart from poultry farming. Broilers (53.3%) were the most common breed of poultry reared and majority (90%) practiced intensive system of farming (Table 2).
Table 1: Demographic characteristics of the poultry farmers. View Table 1
Table 2: Farming characteristics in the poultry farms in ile-ife. View Table 2
All the poultry farmers used one or more antibiotics for their birds which they administered mainly through water (83.3%) (Table 3 and Table 4). The antibiotics used are in different commercial products with a wide variety of trade names (not reported in this study for ethical reasons). Cotrimoxazole (71.7%) and neomycin (66.7%) were the most commonly used antibiotics (Table 4). Resistance of isolates to the antibiotics varied (Table 3). The E. coli strains were more resistant than the staphylococci (Figure 1). E. coli showed 100% resistance to nalidixic acid and amoxicillin, 70-90% resistance to others except gentamicin which gave 50% resistance. Staphylococci isolates showed good susceptibility to tetracycline (90%), moderate to gentamicin and streptomycin (70% and 50% respectively) but are highly resistant to the remaining antibiotics tested (70% and above).
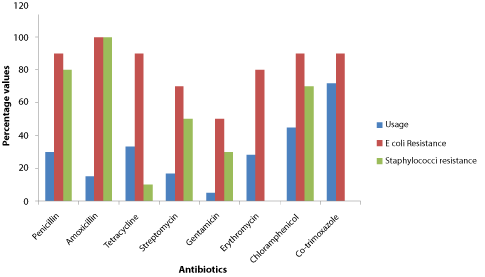 Figure 1: Usage of Antibiotics with their Resistance Patterns of Isolates.
Figure 1: Usage of Antibiotics with their Resistance Patterns of Isolates.
Spearman's correlation (r) showed a weak negative correlation between the use of antibiotics and the development of resistance for Staphylococci (r = - 0.468) and Escherichia Coli (r = - 0.243). View Figure 1
Table 3: Commonly used antimicrobials in the farms and antimicrobial susceptibility of E. coli and staphylococci isolated from the poultry faecal samples. View Table 3
Table 4: Pattern of antibiotic usage in the farms. View Table 4
The antibiotic usage pattern observed in this survey showed that poultry farmers in Ile-Ife relied heavily on antimicrobial medications. Most farms were multi-drug users and all the farms used one or more antibiotics for therapeutic, prophylactic and to a lesser extent for growth promotion. This report is similar to an earlier report that antibiotics were most commonly administered for therapy (36.2%) and prophylaxis (29.3%) among farmers in Ogun State of Nigeria [32]. It was also reported that only about 7% of the farmers used antibiotics for growth promotion. The higher usage of antibiotics for therapeutic and/or prophylactic purposes as observed in this study is also similar to findings on poultry layer farmers in Khartoum State, Sudan [33] and poultry farms in another study in Ogun State, Nigeria [34]. On the other hand, our result is contrary to a report from poultry farms in Ibadan, Nigeria which reported that 86% of the poultry farms used antibiotics for growth promotion [16]. These reports indicate that farmers in different regions of the country used antimicrobials in poultry for varying purposes. This may depend on a lot of factors which may include availability of information, educational levels, scale of farming and financial buoyancy [16,33,34].
Concerning our observation in Ile-Ife, we note that even though a large percentage of the farmers are educated, a larger percentage of the farmers were small scale farmers and may not be financially buoyant to afford continuous addition of antibiotics in bird's feed as growth promoting agents. The dependence of poultry farmers on antibiotics for therapeutic and/or prophylactic purposes may also be due to poor environmental sanitation, unhygienic practices, lack of biosecurity and other management inadequacies leading to increased exposure to bacterial pathogens [35].
The survey also showed that majority of poultry farmers apply antibiotics through oral route by mixing them with the water the poultry (birds) drink. The survey found that cotrimoxazole and neomycin were the most commonly used antibiotics in poultry farms in Ile-Ife. Neomycin is an aminoglycoside that has high activities on Gram negative bacteria [36]. It is used in poultry in cases of bacterial enteritis caused by E. coli and Salmonella causing white diarrhea, paratyphoid and chronic respiratory disease [18,21]. The popularity of cotrimoxazole and neomycin in Ile-Ife could be because of easy availability and affordability of these drugs. In addition, these drugs are water soluble making it possible for oral administration through drinking water. The high rate of oral usage could be the reason for the low use of antibiotics that are mainly administered through the parenteral routes. An example of this is gentamicin which has low usage among the farmers.
The antibiotics used by poultry farmers from various regions in Nigeria varied among studies. Among the most commonly used agents reported in literature included neomycin and gentamicin [37], enrofloxacin and chlortetracycline [24], tetracyclines and sulphonamides [17], fluoroquinolones [32], as well as gentamicin and tetracycline [34].
Based on responses received from respondents, it is evident that most of the poultry farmers do not obtain information on antibiotics they use from qualified personnel. Some of them admitted to relying on directives from drug store vendors while others depended on their own experience for antimicrobial administration. The application of antimicrobials without proper directives amounts to misuse which will eventually be detrimental not only to the chicken but also to public health [36].
All over the world including Nigeria, widespread diseases occur frequently in poultry farms. These diseases occur in all age groups of chickens at any period of time, especially the early stages of life. The economic implications range from weight loss, high mortality rates, carcass downgrade and reduced production [19]. Some of these diseases are associated with resident or ingested proliferation of pathogenic E. coli and staphylococci especially S. aureus [38,39]. When infections occur, different organs or tissues of chickens manifest different signs and symptoms which the veterinarians use to diagnose and treat the infections.
Antibiotics are the drugs used for treating infectious diseases caused by these organisms [40]. After selective pressure resulting from the treatment by antibiotics, bacteria inside the body of diseased poultry can become overwhelmed by resistant strains. These drug resistant organisms can be transferred to humans indirectly through the birds' excretory products in agricultural manure and directly through the food chain [13,39].
Apart from the possibility of these resistant organisms causing drug resistant infections in humans, the transfer of antibiotic resistant genes from these organisms to pathogens and commensals in man has been reported [6,12,38]. It has been shown that major parts of virulence and antibiotic resistance in most bacterial pathogens are acquired characteristics. These characteristics are found in genes embedded in mobile genetic elements such as transposons, bacteriophages, insertion sequences and pathogenicity islands found in these pathogens. These genetic elements can then be transferred intra and interspecies by the processes of transduction, conjugation and transformation [25,36]. These properties have been demonstrated extensively in various pathogenic bacterial strains such as enterococci species, staphylococci species like S. aureus and in species of the enterobacteria such as E. coli [28,36,38]. Their occurrence have serious implications for human health [39].
Antimicrobial susceptibility testing was carried out on the isolated E. coli and staphylococci strains from the ten poultry farms visited. It was found that all the ten E. coli isolates from different poultry farms have developed resistance against nalidixic acid and amoxicillin. Majority had developed resistance against chloramphenicol, cotrimoxazole, cloxacillin, erythromycin, penicillin, tetracycline and ampicillin while half were already resistant to gentamicin. This low resistance rate to gentamicin may be as a result of its low use by the farmers as explained earlier. The few resistance recorded to gentamicin could be a result of cross resistance to neomycin, which is also an aminoglycoside with similar mechanisms of action and resistance. Low level of resistance to gentamicin has been reported in the literature; absolute sensitivity of E. coli (isolated from chicken carcasses) to gentamicin was reported in Jamaica while 17% was reported for those isolated from Portuguese poultry [22]. E. coli strains from four of the ten poultry farms already demonstrated resistance to all the thirteen antibiotics screened.
Although the resistance rates obtained for the staphylococci were not as high as for the E. coli strains, the rates are however still worrisome. While most strains showed susceptibility to gentamicin and streptomycin, higher rates of resistance were obtained for chloramphenicol, erythromycin and the penicillins. Staphylococci strain from one of the poultry farms was already resistant to all the antibiotics screened.
The high resistance to the penicillins despite the low usage rate confirmed the reports of the high prevalence of resistance to them in the study area [41]. Indeed, the low usage could be a result of their low effectiveness.
Our findings provide additional evidence that the poultry production environment in Nigeria represents an important reservoir of antibiotic resistance genes that may spread from livestock production farms to human populations [21].
All the poultry farmers in Ile-Ife used one form of antimicrobial or the other on their poultry. Co-trimoxazole, Neomycin, oxytetracycline, chloramphenicol, tetracycline and penicillin were the commonly used antimicrobial agents and the isolates organisms were found to exhibit high level of resistance to penicillins generally, nalidixic acid, chloramphenicol, and co-trimoxazole.
It is recommended that there should be adequate enforcement of existing veterinary legislations as well as legal classification and control of use of veterinary drugs with the ultimate aim of protecting the public. Also education of the public on the dangers of indiscriminate use of antibiotics and medications especially in poultry and livestock farms is imperative. Farmers should be educated on alternative methods of infectious disease management, such as vaccination, environmental sanitation and disease containment. Responsible authorities should immediately kick off implementation of regulations associated with antimicrobial administrations in poultry production and monitoring programs.
The authors declare that there are no conflicts of interest to declare.
The assistance received from the farmers and drug sellers are appreciated.