Dear Editor,
Pneumocystis jirovecii Pneumonia (PCP) remains an important cause of pneumonia in the Human Immunodeficiency Virus (HIV)-infected patients [1]. Approximately 90% of PCP cases occurred in HIV patients with a CD4 cell count of < 200 cells/mm3, who should receive chemoprophylaxis against PCP early [2]. Compare to PCP infection, Cytomegalovirus (CMV) infection in a HIV-infected patient is often presumed to be non-invasive and does not require therapy but does need close follow-up, as it likes a silent, quiet and dangerous killer [3]. Herein we report on a HIV-infected patient with suspected PCP pneumonia but refractory to specific therapy for PCP. Laboratory data turned out to be negative for PCP but positive for CMV.
This is a 45-year-old man, who has been working in China for several years and denied any systemic diseases before. He has suffered from the right axillary mass for 3 weeks, which was film and associated with mild tenderness. He was admitted to the Hematology department of the hospital on May 1, 2017. The Computed Tomography (CT) scan from neck to the chest showed a large enhancing mass over right axillary area, favoring lymphoma or metastatic lymphadenopathy (Figure 1A). The results of anti-HIV antibody and HIV-1 Western Blot were both positive. HIV viral load was 66,321 copies/mL, and CD4+ lymphocyte count was 139/uL. Meanwhile, the patient developed dyspnea, mild fever, abdominal pain, and renal function impairment with hyperkalemia and metabolic acidosis. Chest X Ray (CXR) film initially showed ill-defined and hazy densities over bilateral lower lung zones (Figure 1B), which later became widespread patchy consolidation over bilateral lungs (Figure 1C). As rapid deterioration of oxygenation, the patient was transferred to the Intensive Care Unit (ICU) on May 5. The incisional biopsy of right axillary mass was then suspended.
At the ICU, the High Flow Nasal Cannula (HFNC) was used. Polymerase Chain Reaction (PCR) for P. jirovecii DNA in the expectorated sputum and other assays for possible opportunistic infections were tested, including Aspergillus galactomannan antigen, blood CMV PCR, sputum Mycobacterium tuberculosis (TB) complex PCR (geneXpert assay), and blood Cryptococcus antigen. As high C-reactive protein (175.4 mg/L) and elevated procalcitonin (3.63 ng/ml) in a blood sample, levofloxacin was used for possible bacterial infections. Meanwhile, sulfamethoxazole & trimethoprim (co-trimoxazole) combined with the standard-dose of adjunctive steroid therapy was initiated for suspected PCP. However, dyspnea persisted and diffuse infiltration in bilateral lungs increased under HFNC support. The Legionella antigen and Pneumococcus rapid antigen screens in urine were negative. Ciprofloxacin was used to replace levofloxacin due to deteriorating pneumonia on May 8. After ten days of treatment, the patient remained intermittently febrile. CXR still showed diffuse infiltration in bilateral lungs (Figure 1D), and dyspnea was worsening. The patient underwent hemodialysis and intubation with mechanical ventilation support for acute respiratory distress syndrome.
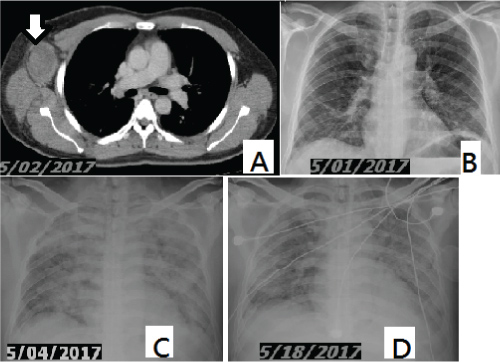 Figure 1: Chest CT scan reveals a large enhancing mass over right axillary area, favoring lymphoma or metastatic lymphadenopathy (A, arrow). CXR shows ill-defined and hazy densities over bilateral lower lung zones (B); Widespread patchy consolidation over bilateral lungs (C); Diffuse infiltration in bilateral lungs (D). View Figure 1
Figure 1: Chest CT scan reveals a large enhancing mass over right axillary area, favoring lymphoma or metastatic lymphadenopathy (A, arrow). CXR shows ill-defined and hazy densities over bilateral lower lung zones (B); Widespread patchy consolidation over bilateral lungs (C); Diffuse infiltration in bilateral lungs (D). View Figure 1
Up to this status, the sputum and blood bacterial cultures of several sets all yielded no growth. The results of Aspergillus antigen, PCR for TB and P. jirovecii and Cryptococcus antigen tests were all negative. Only the result of blood CMV DNA by PCR was positive. Blood Aspergillus antigen and P. jirovecii PCR in the endobronchial aspirates was rechecked. Nevertheless, caspofungin was added as salvage therapy empirically for treating PCP on May 15. Ganciclovir was not given for suspected bone marrow suppression with anemia and thrombocytopenia (38,000/μL on May 18). The condition was getting worse. Severe thrombocytopenia (lowest to 9,000/μL on May 20), acute monocytosis (up to 21.9% on May 22), renal failure, and septic shock developed. The patient received high-dose vasopressors and continuous veno-venous hemofiltration. Thereafter, the condition was still refractory to therapy and the patient died on May 24, 2017. The repeated tests for Aspergillus antigen and P. jirovecii PCR remained negative. Finally, the sputum TB cultures yielded no growth.
CMV infection has been neglected or traditionally regarded as a minimal role in the pneumonia of an HIV-infected patient [3]. The prevalence of CMV viremia detected by PCR in patients with HV infection, especially in those with advanced stages of immunosuppression, is high [4]. About 30% to 55% of HIV infected patients with < 100/uL CD4+ lymphocytes had detectable CMV viremia [5,6]. Despite the high proportion of patients with CMV viremia, only few of them develop end-organ-disease due to CMV. Therefore, CMV reactivation has been ignored in the current case. However, the risk of CMV end-organ disease increases (hazard ratio 12.6; 95% confidence interval 4.3-37.4) if CMV DNA is detected in the plasma of HIV-infected patients [5], which is also associated with increased mortality [6]. Besides, our patient had a CD4 count of > 100/uL and thus detection of CMV viremia should be rather uncommon. Moreover, adjunctive steroid therapy during therapy for PCP might potentiate the pathogenic role of CMV infection, which may be responsible for failing treatment of severe PCP [7]. We also reported an HIV-infected patient who had second hit of pneumonia by CMV after initially successful therapy for PCP [8].
Co-trimoxazole has been the drug of choice to treat PCP and a combination of caspofungin with low-dose co-trimoxazole was reported to treat severe PCP successfully [9]. Although negative results of P. jirovecii PCR testing in the expectorated sputum and endobronchial aspirates without performing bronchoalveolar lavage could not completely rule out PCP diagnosis, our case experienced a different clinical entity with failure of initial co-trimoxazole and subsequent caspofungin therapy, suggesting other etiologies than PCP.
In conclusion, CMV may be a contributor or a bystander in the pneumonia of an HIV-infected patient. The presumptive diagnosis of CMV pneumonia in current case was based on 3 facts: 1) The lack of response to P. jiroveci treatment, 2) The detection of CMV viremia and 3) All the other important diagnostic clues for immunocompromise-related infections were negative. The manifestations of thrombocytopenia and monocytosis (22%) may also favor the CMV syndrome. Therefore, we should have treated the patient as CMV pneumonia earlier. Our report reminds physicians that CMV might paly an important role throughout the whole course of pneumonia in a HIV-infected patient, especially negative for PCP. The limitation of the report was lack of bronchoalveolar fluid or lung tissue histopathology to confirm the CMV diagnosis.
Authors declare no conflict of interests for this article.
None declared.