Despite the widespread availability of antiretroviral therapy, Cytomegalovirus (CMV) infection remains an important cause of morbidity and mortality in sub-Saharan Africa. CMV gastrointestinal tract infection is poorly described. This retrospective study was undertaken to describe the clinical and histological features of biopsy confirmed CMV infection of the gastrointestinal tract in a large teaching hospital with a high prevalence of Human Immunodeficiency Virus (HIV) infection. A total of 53 tissue biopsies from 47 patients and 58 topographic sites were included. Forty (91%) adults had a predisposing condition; 32 (73%) were HIV infected. The median adult age was 41 years and 61% were female. The sites involved were oesophagus (35%), colon (24%), small intestine (21%), stomach (14%) and anus (7%). Four patients had intestinal perforation. Ulcers were the most frequent endoscopic finding but 25% had 'pseudotumours'. No relationship was noted between the degree of inflammation and the load of CMV infected cells assessed microscopically. Endothelial cells were most commonly infected and Cryptosporidium and Helicobacter were the commonest co-pathogens. This study highlights important features of CMV infection in the gastrointestinal tract especially with regard to HIV infected patients who formed the majority of our cohort.
Cytomegalovirus, Gastrointestinal tract, South Africa
Cytomegalovirus (CMV), historically believed to only cause rare congenital infections, has emerged as an important pathogen over the past few decades with the increase in post-transplant and Human Immunodeficiency Virus (HIV) infection immunosuppressive states. Due to the high prevalence of HIV infection, CMV infection and disease is expected to be common in Africa. CMV seroprevalence in a cohort of South African HIV infected, ART naive patients was 100% [1]. Other risk factors for CMV gastrointestinal tract (GIT) disease include malignancy, inflammatory bowel disease, steroid use, rheumatological disorders such as lupus, and advanced age [2]. Three recent reviews on CMV in Africa have noted the paucity of studies on extraocular disease. The need for endoscopically directed biopsies for diagnosis may be a limiting factor [3-5]. Fourteen percent of HIV-infected adults with abdominal pain had CMV disease [6]. Of the 38 children with GIT CMV disease 55% were HIV infected and oesophageal and small bowel disease were commonest [7]. GIT symptoms, abnormalities on endoscopy and histological detection of CMV infection in a GIT biopsy specimen are required to diagnose CMV GIT disease. CMV may be deduced as the aetiological agent once other causes for ulceration/erosion have been excluded [8]. Typical histological features of CMV infection are cell enlargement and intranuclear inclusions but atypical features such as eccentric or smudged nuclei and perinuclear amphophilic zones may also be noted [9]. CMV can be detected by culture, immunohistochemistry (IHC) on infected tissue, pp65 antigen testing in peripheral blood and quantitative nucleic acid testing by Polymerase Chain Reaction (PCR). Recommendations for CMV monitoring post-transplant are in place but clear guidelines are lacking for HIV infected individuals [10]. Currently used tests do not predict CMV disease well [5]. This study was undertaken to describe the spectrum of histologically confirmed CMV GIT infection in Soweto, South Africa, an area of high HIV seroprevalence.
The study was based at Chris Hani Baragwanath Academic Hospital (CHBAH), a 2800-bed public sector university hospital which serves the population of Soweto and surrounding areas. It has limited oncology services and no transplant programme.
A retrospective descriptive study was conducted. All cases of CMV diagnosed on GIT tissue biopsy between 2007 and 2014 were retrieved using search programs of the laboratory's DISA and Trakcare electronic systems. The original Haematoxylin and Eosin (H&E) slides were reviewed by a histopathologist (RDM) and morphological features of CMV infection such as nucleomegaly and intranuclear and intracytoplasmic inclusions were confirmed on re-examination. The CMV IHC slides where available were reviewed and stains recorded as positive or negative. Specific CMV infected cells noted on H&E stained sections were matched with the IHC slides. The presence of an additional aetiopathogen was recorded. The degree of inflammation was graded as severe if accompanied by ulceration, mild if focal inflammatory cells were noted and moderate if there were diffuse inflammatory cells without ulceration (similar to criteria used by Yan, et al. [9]. CMV infected cells were quantified as scanty (isolated infected cells, 1-3 per slide), moderate (> 3 infected cells per slide) or heavy (easily identifiable inclusions, numerous and in many fields) on H&E slides. Infected cell types were recorded based on their morphological appearance and their location in the tissue biopsy. Smooth muscle cells were identified as being located in the muscularis mucosa or propria and had elongated cigar shaped nuclei and abundant eosinophilic cytoplasm. Endothelial cells lined spaces filled with blood and epithelial cells were identified on the surface or within crypts. Fibroblasts were stellate spindle cells usually located within the ulcer base.
Clinical information was retrieved from patient files (by AK). This included age, gender, presenting symptoms and signs, HIV serological status, CD4 counts, predisposing conditions, management, treatment and outcome. CMV serological and PCR testing were inconsistently performed and limited to very few patients, therefore these variables were excluded. Where clinical files could not be located or were incomplete, data were obtained from the laboratory database and the case histories accompanying the histopathology request form.
All information was captured electronically and was subsequently analysed using Statistica 13. Categorical data were assessed as frequencies and ratios and continuous data have been presented as mean ± SD if normal distributed and as median and interquartile range (IQR) when the distributions were non-normal. The chi square test was used to assess the relationship between categorical variables and the level of significance was set at p = 0.05.
Permission to access patient files was obtained from the CHBAH Medical Advisory Committee and ethics approval was granted by the Human Research Ethics Committee (medical) at the University of the Witwatersrand (certificate clearance no. M150670).
The total number of biopsies seen at the histopathology laboratory over the 8-year time period was 135497. Fifty-three (0.04%) of these biopsies showed CMV GIT infection and were reviewed. The biopsies were from 47 patients (44 adults and 3 children). Some patients had multiple areas biopsied resulting in 58 gastrointestinal tract sites being represented. Four patients were biopsied on more than one occasion. The number of patients with CMV per GIT site were, in order of frequency, oesophagus 20 patients (35%), colon 14 (24%), small intestine 12 (21%), stomach 8 (14%), and anus 4 (7%). The baseline clinical characteristics are detailed in Table 1. Clinical files could not be found for 12 patients. There was a female preponderance among adults with a 1.6:1 Female: Male ratio. The median age in adults was 41 (IQR 32-50) years. Eight patients were older than 55 years. Forty adults (91%) had a predisposing condition, 32 (73%) were HIV seropositive. The median CD4 count was 26/mm3 (IQR 7-76/mm3) for the 30 patients that were tested. Twenty patients (66.7%) had CD4 counts less than 50/mm3 and 6 (20%) had CD4 counts > 100/mm3. Thirteen patients (41%) were on antiretroviral therapy, 4 had virological failure and 2 defaulted treatment. Of the 5 (11%) HIV seronegative patients, 2 had ulcerative colitis, 1 had chronic diarrhoea due to suspected inflammatory bowel disease, 1 had lupus and was receiving corticosteroids and 1 had carcinoma of the gallbladder. All seven patients not tested for HIV infection were older than 50 years. Altogether, five patients had diabetes mellitus.
Table 1: Baseline clinical characteristics in 47 patients with CMV GIT disease. View Table 1
Three children were diagnosed with CMV infection on tissue biopsy; two were HIV seropositive with CD4 counts of 28/mm3 and 49/mm3. An 11-month-old presented with an acute abdomen secondary to an ileal perforation. A 3-month-old presented on numerous occasions with severe oesophagitis and a distal oesophageal stricture. No co-pathogens were noted on histology in the paediatric cases and all children were discharged home with no record of death.
Patients with oesophageal CMV had dysphagia (n = 9) and/or odynophagia (n = 8). Gastric and small intestinal CMV manifested as pain (n = 7), vomiting (n = 8) and weight loss (n = 9) and colonic CMV with diarrhoea (n = 7). Four patients presented with intestinal perforation, two of whom died. Ulceration was the most common endoscopic finding (n = 23). Nine patients of those with recorded endoscopic findings (25%) had exophytic masses/polyps.
Documentation of ganciclovir treatment was inconsistent and difficult to interpret. At least 10 patients were treated. Thirteen patients (27.7%) died within 30 days of admission, 11 of these patients were HIV seropositive with a median CD4 count of 51/mm3 (IQR 7-52), 3 (23.08%) also had non-Hodgkin lymphoma. The HIV seronegative individual had a severe fixed drug reaction and co-morbid epilepsy. Of those that died, 3 (23.08%) had co-infecting pathogens and colon (n = 5) was most commonly infected.
The histological findings are presented in Table 2. Most biopsies (n = 40) showed severe inflammation with ulceration. Scanty CMV infected cells were noted in 30 biopsies, moderate in 15 and heavy loads were noted in 13 biopsies (Figure 1). There was no correlation between the quantity of CMV infected cells and the degree of inflammation (p = 0.828). The cell type most frequently infected was endothelium (n = 50). Of the 5 cases with infection of smooth muscle, 3 had bowel perforation (Figure 2). Crypt epithelial cells were infected in seven cases and surface mucosae were never infected. CMV IHC was performed in a total 31 biopsies (53.5%), 32.3% of these stains were negative. All IHC negative cases had scant CMV inclusions on H&E that were cut away on the level for IHC. An additional aetiopathogen was noted in 8 (13.8%) biopsies. Cryptosporidium was noted in one case of CMV in the colon, one in the stomach (Figure 3) and another in the small intestine. Helicobacter were noted in 3 gastric biopsies that also showed evidence of CMV infection. One oesophageal biopsy also showed Candida and a small intestinal biopsy showed mycobacterial infection.
Table 2: Histologic characteristics on review. View Table 2
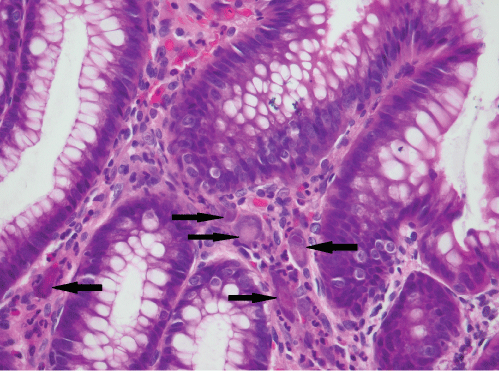 Figure 1: Heavy CMV load with many inclusions identified (original magnification, x400).
View Figure 1
Figure 1: Heavy CMV load with many inclusions identified (original magnification, x400).
View Figure 1
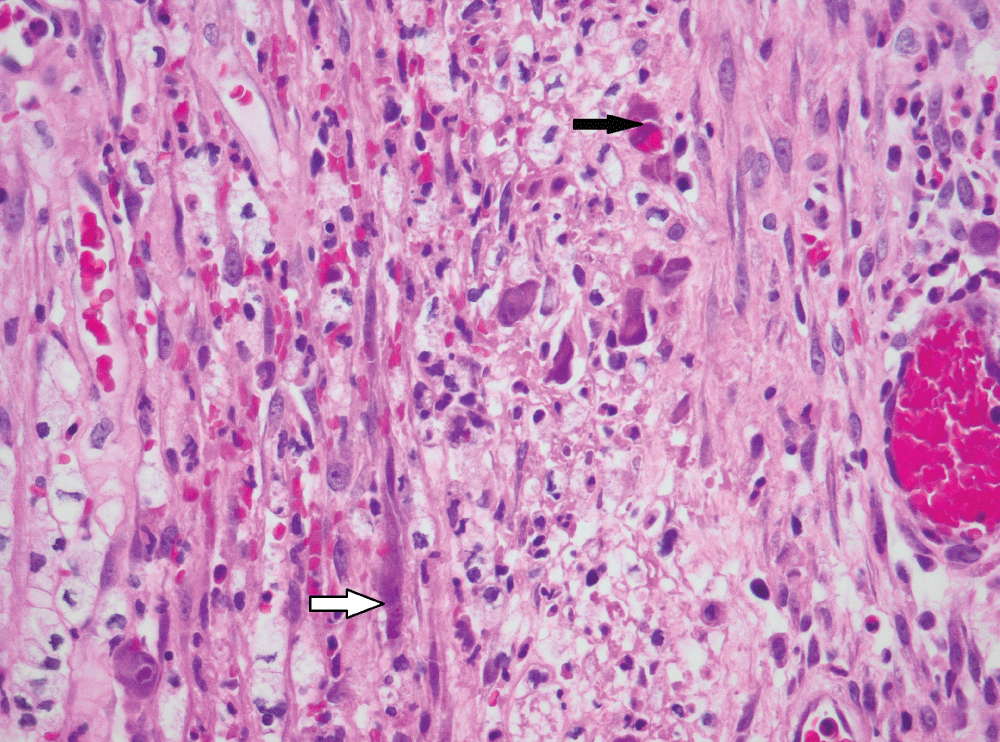 Figure 2: CMV infection of smooth muscle cells (white arrow) and endothelial cells (black arrow) in bowel removed for perforation (original magnification, x400).
View Figure 2
Figure 2: CMV infection of smooth muscle cells (white arrow) and endothelial cells (black arrow) in bowel removed for perforation (original magnification, x400).
View Figure 2
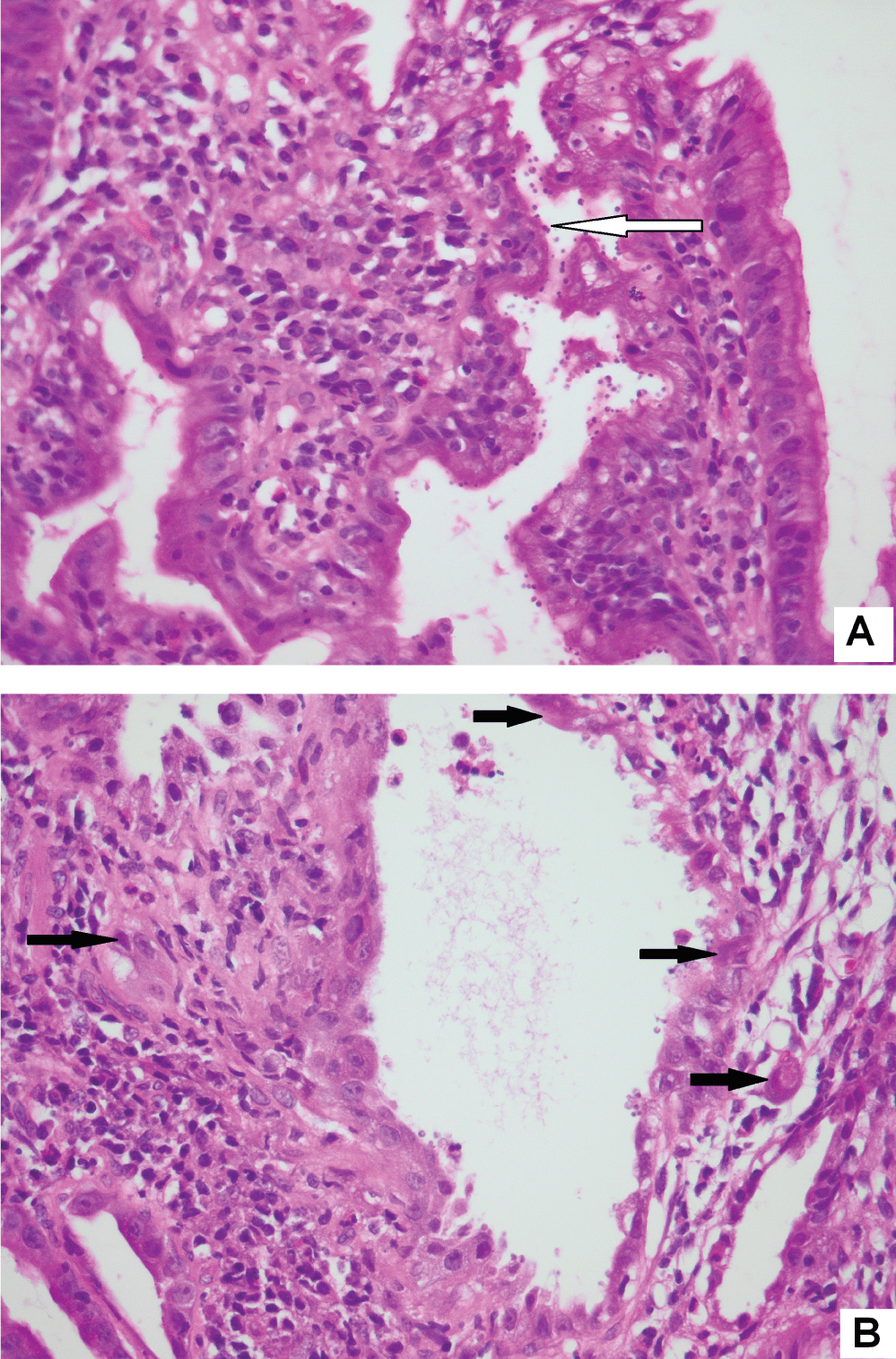 Figure 3: Same gastric biopsy with both Cryptosporidium (3A) and CMV infection (3B) (original magnification, x400).
View Figure 3
Figure 3: Same gastric biopsy with both Cryptosporidium (3A) and CMV infection (3B) (original magnification, x400).
View Figure 3
With the widespread introduction of ART, cytomegalovirus infection has declined [11]. There are few studies especially from Sub Saharan Africa on CMV infection of the GIT due to difficulties in confirming the diagnosis. An autopsy study found CMV infection of the GIT in 29% of cases [12], this may indicate underdiagnosis. The GIT sites more frequently diagnosed with CMV in our study were oesophagus and colon similar to other studies [13,14]. It is expected that oesophagus and colon are the commonest sites of documented CMV infection as odynophagia or dysphagia and chronic diarrhoea, the usual presenting symptoms, are debilitating and mandate investigation and management. Given the high HIV seropositivity in our cohort, the finding of a greater prevalence of CMV infection in females may be reflective of the increased HIV seropositivity in young females who bear the brunt of this disease in sub-Saharan Africa. Ninety-one percent of patients had a predisposing disease and HIV infection with low CD4 counts was the commonest predisposing disease. All 5 patients who were HIV non-infected also had a predisposing condition. In a meta-analysis of CMV in immunocompetent people, it was found that patients with no co-morbidities had the highest rate of spontaneous remission and that mortality was highest in those with immune modulating conditions [15]. There were 8 patients in our cohort older than 55 years. Patients older than 55 years have co-existing immune modulating conditions and aging weakens the immune system [15]. Thirteen patients were on ART prior to hospitalization; almost half had virological failure or had stopped therapy. The failure of ART in those presenting with GIT opportunistic disease has been noted in the United States [11]. Two thirds of patients who were tested had CD4 counts < 50/mm3 in keeping with another study [16]. Fewer paediatric cases were noted compared to another South African study [7]. This may reflect regional differences in management practices. There could be cases at this hospital that were not biopsied at time of endoscopy. Symptoms were similar to those reported elsewhere [7,8,17]. Dysphagia, abdominal pain, loss of weight and diarrhoea are more frequent in patients with CMV compared to other infections [18]. The symptoms are often non-specific and are open to a broad differential diagnosis. In patients with chronic diarrhoea and negative stool cultures, the endoscopic diagnostic yield was 37% and CMV was the most commonly identified pathogen [19].
Pseudotumours as an endoscopic finding for CMV GIT infection have been reported by others and are postulated to be due to infection of stromal and epithelial cells resulting in hyperplastic changes [17,20]. Mortality was high (27.7%) and predominantly in HIV infected patients with low CD4 counts, but attribution solely to CMV disease was not always clear. A mortality rate of 32% in a paediatric cohort was reported, half of all deaths occurred within one month [7]. Decreased mortality is achieved with improved body weight, higher CD4 counts, higher hemoglobin levels and lower viral loads, all attainable by ART usage [14]. We found Cryptosporidium and Helicobacter to be the commonest co-pathogens. Other co-infecting pathogens that have been described include Mycobacterium (M. avium), adenovirus, Entamoeba and candidiasis [7,18,21]. One of these studies [18] found co-infection with CMV in 37% of cases. Confirmation by IHC is useful [9] but should not be performed reflexly or at the request of the gastroenterologist due to cost [22]. There should be a high index of suspicion of CMV infection in biopsies from immunosuppressed patients showing significant inflammation.
CMV infects endothelium, macrophages, crypt epithelium, fibroblasts and smooth muscle cells. Similar to another report [13], surface and squamous epithelia were never infected. CMV infection of endothelial cells and macrophages accounts for haematogenous spread [23]. CMV endothelial cell infection is associated with dense infection and acute deterioration. It causes vascular luminal narrowing and a decrease in blood flow [24].
We found no correlation between the CMV infected cell load and the degree of inflammation similar to another study [9] that also showed no correlation between the blood viral load and infected cell counts. Treatment includes ganciclovir and valganciclovir. Foscarnet is used for resistant CMV strains. Therapy may be useful in symptomatic individuals even if only isolated CMV infected cells are noted on biopsy. Two of eight patients with isolated CMV infected cells who were not treated died following worsening of disease [9]. The mortality rate in our cohort was high, just over one quarter of patients died within 30 days of admission.
Unavailable and incomplete clinical records were limitations of this retrospective study. The lack of serological testing for CMV viral load did not allow for assessment of association with tissue load. Software was not available to count the number of infected cells and CMV IHC was not performed for all cases.
As there are only a few papers on histologically confirmed CMV infection in the GIT, this study provides some important insights. In immunosuppressed patients with GIT symptoms, a high index of suspicion must be maintained by the clinician and histopathologist. If all other causes for symptoms have been excluded in the presence of CMV inclusions, CMV may be considered the underlying aetiological agent and not merely a bystander. Timely diagnosis and initiation of treatment is vital to reduce mortality. The histopathologist can assist in documenting the quantity and type of infected cells and the degree of inflammation.
We thank Ms Hellen Nxumalo for conducting the database search for cases, Mr. Eric Liebenberg for assistance with photography, general surgeons and gastroenterologists for biopsying, Mr. L. Nyati of the Faculty Research Office Biostatistic Support and my colleagues Dr's. S Pather and E van den Berg.
None.