Preeclampsia (PE) remains a leading cause of maternal and neonatal morbidity and mortality globally. Among several experimental models developed to interrogate the pathogenesis of PE, the mouse model employing systemic infusion or transgenic overexpression of soluble fms-like tyrosine kinase-1 (sFlt1) has gained widespread use due to its capacity to induce cardinal features of the human disease. These include maternal hypertension, renal injury, endothelial dysfunction, placental abnormalities, fetal growth restriction, and adverse long-term outcomes. This review critically evaluates the sFlt1-based mouse model of PE, highlighting its utility for understanding the pathogenesis of angiogenic imbalance and its sequelae. We contrast findings from this model with clinical observations in human PE and discuss applications for studying early-onset versus late-onset forms. Finally, we address limitations and propose strategies to enhance its translational relevance. By situating the model within the context of human disease, this review informs its optimal use in future preclinical and translational research.
Preeclampsia, sFlt1, Mouse model, Hypertension, Fetal growth restriction, Placenta, Translational research
Preeclampsia (PE) is a multisystem pregnancy disorder characterized by new-onset hypertension and proteinuria after 20 weeks of pregnancy, due to placental dysfunction and often accompanied by fetal growth restriction (FGR). The condition contributes substantially to global maternal and neonatal morbidity and mortality. Central to PE pathophysiology is an imbalance in angiogenic factors-particularly an excess of anti-angiogenic soluble fms-like tyrosine kinase-1 (sFlt1). The sFlt1 protein is a splice variant of the VEGF receptor that lacks the trans membrane domain and the tyrosine kinase domain that allows for VEGF angiogenic signaling. sFlt-1 binds to and sequesters circulating VEGF and placental growth factor (PlGF, a truncated VEGF homolog secreted by the placenta), impairing endothelial health and trophoblast function [1,2]. Levels of sFlt1 increase throughout normal pregnancy but increase faster and to a greater level in PE pregnancies [3].
Animal models have advanced our understanding of PE, with sFlt1-based mouse models emerging as especially useful due to their ability to reproduce many PE-like features [4-6]. Here, we evaluate this specific model's ability to mimic human disease and its translational applications, including relevance to early- versus late-onset PE, and discuss therapeutic insights it may offer.
The sFlt1 mouse model is implemented via adenoviral vector-mediated gene delivery, protein infusion, or genetic overexpression (Figure 1) [1,5,6]. Systemic infusion beginning around gestation day 8.5 of recombinant sFlt1 protein or viral vectors targeting maternal tissues leads to acute elevations of circulating sFlt1 during mid-to-late gestation. Alternatively, transgenic models using trophoblast-specific expression systems (for example, the transgenic inducible human sFlt1/reverse tetracycline-controlled transactivator (hsFLT1/rtTA) mice, in which human sFlt1 is ubiquitously overexpressed during pregnancy in dams) offer spatiotemporal control and more gradual sFlt1 elevation [5,6].
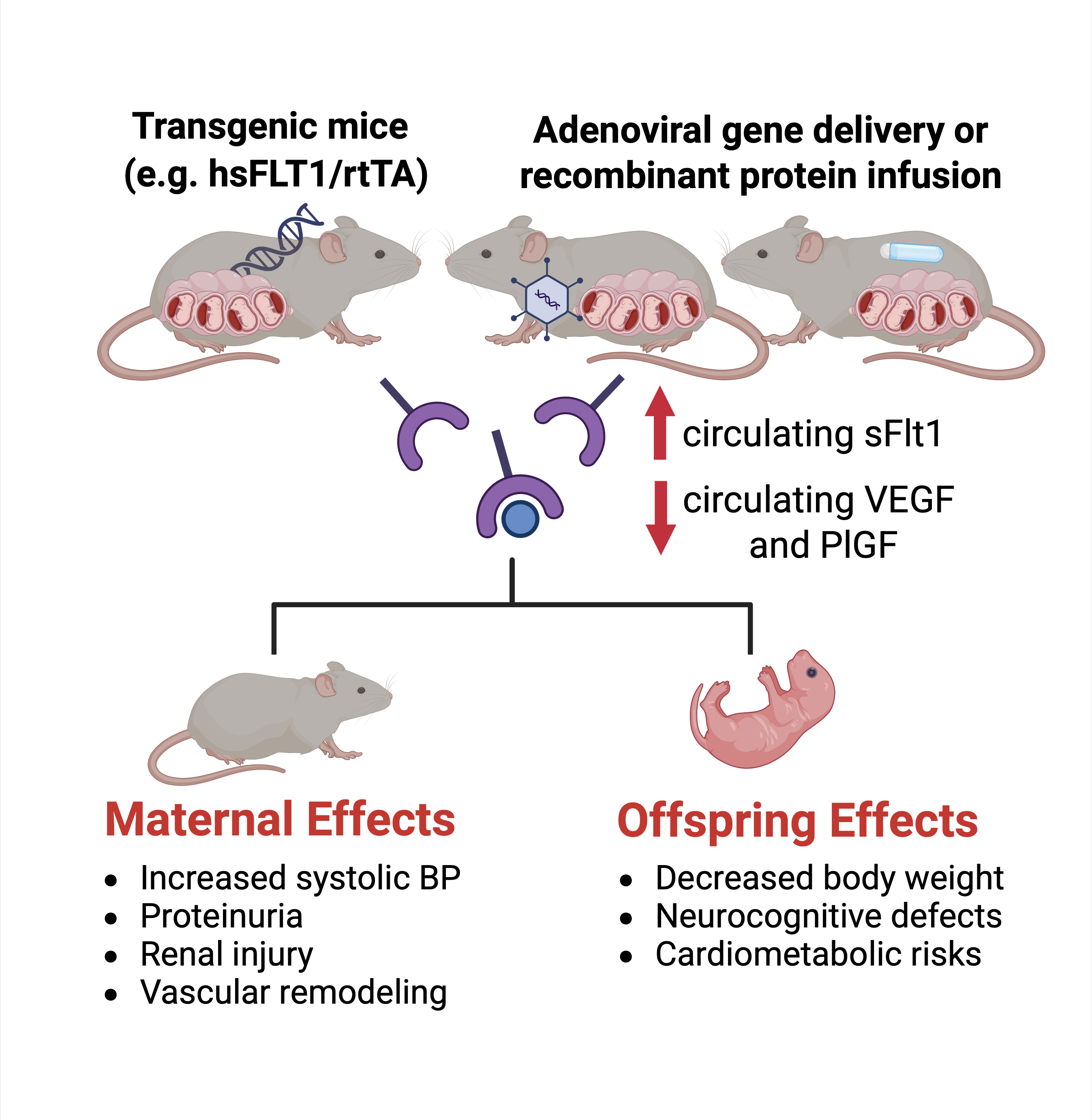 Figure 1: Schematic of the sFlt1 Mouse Model of Preeclampsia.
Figure 1: Schematic of the sFlt1 Mouse Model of Preeclampsia.
sFlt1 can be overexpressed by transgenic approaches or by systemic infusion of viral vectors targeting maternal tissues or sFlt1 recombinant protein. This leads to increased circulating sFlt1 and decreased circulating VEGF and PlGF. This model recapitulates maternal and offspring effects seen in humans. (Created with Bio render).
View Figure 1
These approaches differ in onset kinetics, dose control, and tissue specificity, influencing the degree of hypertension, proteinuria, and placental changes. Importantly, infusion models allow fine-tuned temporal study of sFlt1 effects, whereas transgenic models facilitate exploration of early placental development [5].
sFlt1 overexpression in pregnant mice leads to elevated systolic blood pressure, with severity dependent on dosage and timing [1,7]. Proteinuria accompanies the hypertensive phenotype, mirroring clinical PE. Renal histopathology often reveals glomerular endotheliosis and podocyte injury, consistent with findings in human PE (Figure 1) [1,2].
sFlt1 impairs placental vascularization, particularly in the labyrinthine layer, and induces apoptosis in trophoblasts [5,6]. These alterations reduce nutrient exchange and result in asymmetric FGR. Observed fetal changes include decreased body weight and increased brain-to-liver weight ratios [4,6]. Some studies also report cortical disorganization and caudate putamen density changes, suggesting developmental impacts paralleling neurocognitive risks seen in PE offspring (Figure 1) [4].
Endothelium-dependent vasorelaxation is diminished in sFlt1-expressing mice [8]. There are unpublished data suggesting increased vascular reactivity to vasoconstrictors, with emerging evidence implicating the MAPK/ERK pathway [9]. Aortic remodeling and increased arterial stiffness have been documented postpartum, recapitulating heightened cardiovascular risk among women with prior PE (Figure 1) [10-12].
Male offspring of sFlt1-overexpressing pregnancies demonstrate greater susceptibility to insulin resistance, weight gain, and hepatic gene expression changes (Figure 1) [13,14]. Epidemiological data from humans suggest there might be similar sexual dimorphism in the risk for adverse cardio metabolic programming after PE pregnancies, but studies are not consistent [15-17].
The model facilitates preclinical testing of angiogenic, anti-inflammatory, and redox-modulating agents. Recombinant VEGF and PlGF have shown reversal of hypertension and improved fetal outcomes [7]. Nitric oxide donors and agents such as AKT-1005 reduce oxidative stress and blood pressure [18]. Progesterone-induced blocking factor (PIBF) has shown promise in improving placental mitochondrial function [19]. These interventions highlight the model's utility for testing pathogenesis-targeted therapies.
The model most accurately recapitulates early-onset PE, marked by angiogenic imbalance and severe placental disease [6,20]. Late-onset PE, often more heterogeneous and influenced by maternal metabolic and cardiovascular comorbidities, is less faithfully modeled. Incorporation of maternal stressors (e.g., high-fat diet or advanced maternal age) into sFlt1-overexpressing backgrounds may better approximate the latter phenotype [14].
While sFlt1 models recapitulate hallmark features of early-onset PE, they are limited by lack of immunological complexity and absence of maternal-fetal immune interaction studies. Hemodynamic parameters beyond blood pressure (e.g., cardiac output, uterine artery Doppler indices) are underexplored. Further, acute induction of sFlt1 may not mimic gradual pathological progression [2,6]. Lastly, hypertension and proteinuria resolve for most women after delivery in the absence of placental secreted vascular factors [21,22]. While blood pressure and vascular reactivity has been reported to normalize 6-8 months postpartum (equivalent to decades in humans), the temporal resolution of vascular and renal function immediately after delivery in the sFlt1 mouse is not well described.
Combining sFlt1 overexpression with other models-e.g., reduced uterine perfusion pressure (RUPP), inflammation-driven models, or genetic mutants-could help simulate multifactorial PE. Integration of omics approaches (transcriptomics, proteomics) may also uncover novel pathways and biomarkers [13,20].
The sFlt1 infusion and transgenic overexpression mouse models offer critical insights into the pathogenesis of angiogenic imbalance in PE. They reliably reproduce hypertension, renal injury, placental insufficiency, and fetal growth restriction-especially relevant for early-onset disease. These models are valuable platforms for therapeutic testing but require refinement and complementation to fully capture PE heterogeneity. A comparison of key features of human PE and those of the sFlt1 infusion and transgenic overexpression mouse PE model is found in the (Table 1).
Table 1: Comparison of sFlt1 Mouse Model with Human Preeclampsia. View Table 1
As PE continues to be a significant global health burden, optimizing translational animal models remains essential. The sFlt1 mouse model represents a key tool in this endeavor, especially when integrated into a broader preclinical research framework.