Current guidelines for colorectal cancer recommend starting screening at age 50 until 75 years. The primary objective was to compare survival in patients 50-74 years-old versus older patients. Retrospective chart reviews were performed on patients who underwent screening, diagnostic or surveillance colonoscopy at JAL FHCC. 213 patients were included with 51% of the patients aged 50-74 years and 49% aged 75 years or older. Survival time was higher in the younger group with mean survival time (MST) of 10.9 years compared to 8.9 years in the older group (p = 0.009). The highest MST of 11.6 years was observed in patients aged 50-74 years who had screening colonoscopy while lowest MST of 7.8 years was seen in patients over 75-years-old who had diagnostic colonoscopy (p < 0.001). Although patients 50-74 had statistically significant higher MST, older patients also had increased MST of 8.9 years which may impact screening recommendations.
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in both men and women. In 2019, there were an estimated 145,600 cases of colorectal cancer in the US. Additionally, there were an estimated 51,020 cases of mortality due to colorectal cancer in the US [1]. This improvement in mortality statistics can be attributed to more effective screening. Early detection of colorectal cancer plays an important role in ensuring disease free survival. Even though colonoscopy is an effective screening tool, the potential benefits can vary substantially according to a patient's age and chronic disease burden. As per current screening guidelines (ACS, NCCN, USPSTF), screening for colon cancer should begin by the age of 50 and be continued till the age of 75. Routine screening over 75 years is not recommended. Decision modelling studies supported the above guidelines [2]. Our literature review failed to reveal any studies evaluating impact of colonoscopy based on actual survival times in older age group. We conducted a retrospective chart review to compare survival times in different age groups to help determine impact of performing colonoscopy in persons aged over 75 years.
The primary objective of the study focuses on comparing (survival of) patients who are under 75-years-old versus those who are older or equal to 75-years-old (who had undergone colonoscopy for diagnostic, screening or surveillance). In our main research hypothesis, we suggest there are differences in overall survival between the two patients' groups and the proportion of patients with pathological findings is higher in patients older than 75.
The study was performed in Captain James A. Lovell Federal Health Care (FHCC) which is the only hospital that provides care to veterans and Department of Defense beneficiaries. Retrospective chart reviews of patients who were 75 years or older and underwent colonoscopy for screening, diagnostic or surveillance purposes, as well as of those patients who were between age of 50 and 74 years with similar procedure indications were performed. Patient records from FHCC were collected from inpatient and outpatient settings between 2008 and 2012. Exclusion criteria include known GI malignancy, life expectancy less than 5 years and age less than 50 years. The data collected were later de-identified to establish patient's privacy in conjunction with HIPAA policy. The authors have no conflict of interest. No benefit to patients was provided. IRB approval was obtained before the study started.
The following demographics parameters were collected (Table 1): Age, sex, race, and ethnicity; risk factors including smoking status and alcohol use; comorbidities including diabetes mellitus, hypertension, chronic kidney disease (CKD), coronary artery disease, atrial fibrillation, history of stroke, dyslipidemia with use of statin and dementia.
A comorbidity index or modified Charlson Deyo Score was used in our data analysis to estimate overall risk of patients based on their comorbidities. We used National Comprehensive Cancer Database dictionary as a reference for our Charlson Deyo score with a zero score for patients with zero risk factors. A score 1 was given to patients who have one comorbidity and a score of 2 was given to those with more than one comorbidities. Colonoscopy indications were captured in the study as listed above. Procedure related complications were also captured.
Pathological findings were collected and divided into high grade lesion (HGL) and low grade lesions. HGL were defined as adenomas > 1 cm, tubulovillous polyps, villous polyps, high-grade dysplasia or cancer. Otherwise, abnormal findings that do not meet the above criteria were reported as low grade lesions.
Survival time was calculated based on the time since colonoscopy was done until date of death or last contact or follow up. Patients who were lost to follow up were censored in survival analysis accordingly. A total of 213 patients' records were reviewed, patients were divided into two groups, 108 who were younger than 75-years-old and 105 who were older or equal to 75-years-old.
The data analysis was based on the comparison of the two age groups (those who were younger than 75-years-old versus those who were older or equal to 75-years-old). The survival analysis was performed by Kaplan Meier test with log-rank (Mantel-Cox) (Figure1) statistics to compare the mean survival time between the two groups. Categorical variables such as patients' demographics, comorbidities or pathological findings were analyzed by Pearson Chi-square test or Fisher's exact test when appropriate. Continuous variables were analyzed by independent sample t-tests. Binary logistic regression test was employed to find predictors for high grade lesions. Statistical analyses were performed by IBM SPSS software package (version 22). Differences were considered statistically significant with a "p" value of < 0.05 (two sided alpha error < 0.05).
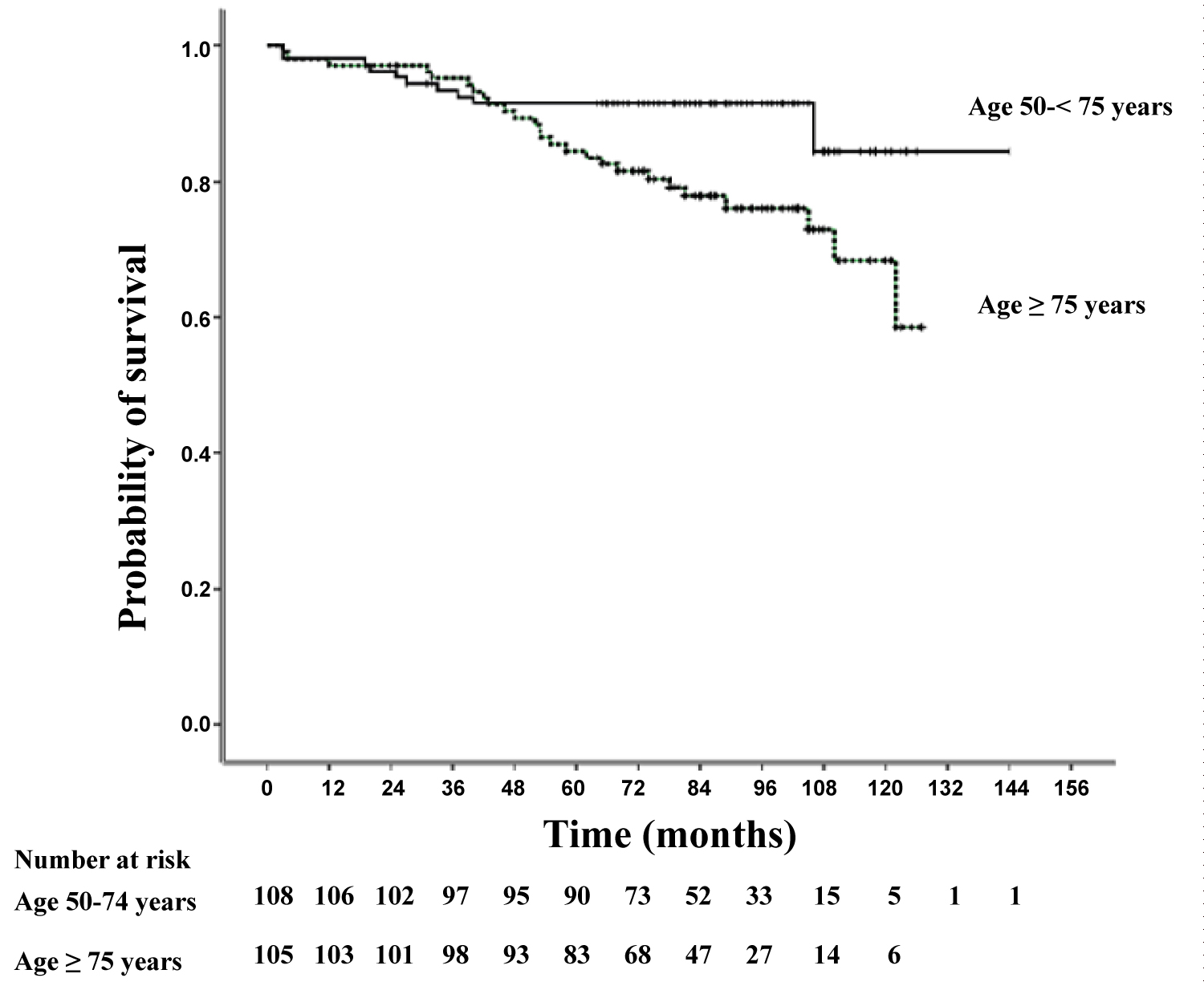 Figure 1: Kaplan-Meier estimates of the probability of survival from the time of colonoscopy.
Figure 1: Kaplan-Meier estimates of the probability of survival from the time of colonoscopy.
Patient in the older age group had significantly shorter survival than patients in the younger age group (p = 0.009).
View Figure 1
A total of 213 patients were included in the study (Table 1). Fifty one percent of the patients (108) were 50-74 years-old (young age group), while 49% (105) were 75 or older (old age group). Alcohol use and smoking were more prevalent in younger age group, while there were more comorbidities in the older age group.
Table 1: Patient's demographics. View Table 1
Patients had colonoscopy for the following indications (Table 2): 92 (43%) screening colonoscopy, 62 (29.1%) diagnostic colonoscopy and 59 (27.7%) surveillance colonoscopy. There was no statistically significant difference between the age groups in the indications of colonoscopy (p = 0.899).
Table 2: Indications and Results. View Table 2
The difference in any pathological findings between the two groups was not statically significant (p = 0.650). High grade lesions were defined as adenomas > 1 cm, tubulovillous polyps, villous polyps, high-grade dysplasia or cancer. Among those patients who underwent screening colonoscopy, 8 of the 44 older patients (18%) had high grade lesions compared to 4 of the 48 younger patients (8.3%), indicating a higher proportion of high grade lesions in older patients, although the difference was statistically not significant (p = 0.161 a trend).
In the entire cohort, history of CKD (p = 0.011) and Charlson Deyo score (p = 0.012) were independent predictors of high grade lesions, while age was not a predictor (p = 0.964).
The mean overall survival time for all patients was 123.6 months (10.3 years). Survival time was significantly higher in the young age group with a mean survival time of 131.1 ± 3.7 months (10.9 years) compared to 106.9 ± 3.5 months (8.9 years) in the old age group (p = 0.009). The highest mean survival time was observed in patient who were under 75-years-old and had colonoscopy for screening purposes (138.9 ± 3.5 months, 11.6 years) and the lowest mean survival time was seen in those who were more than 75-years-old and had colonoscopy due to diagnostic purposes (93.9 ± 6.8 months, 7.8 years), p < 0.001.
Many previous studies have attempted to define the age at which screening colonoscopy carries more risks than benefits by comparing CRC incidence rate and adverse events rate related to the procedure itself. We have carefully reviewed the most recognizable guidelines and summarized their recommendations in our discussion part. We also presented our results and explained their impact on those guidelines.
The National Comprehensive Cancer Network (NCCN) guidelines, 2018 version, recommend CRC screening in adults in the age range of 50-75 years. The decision to screen between ages 76-85 years should be individualized and should include a discussion of the risks and benefits based on comorbidity status and estimated life expectancy [3].
The American Cancer Society (ACS) recommends colorectal cancer screening between ages 45-75. For people aged 76 through 85, the decision to be screened should be based on a person's preferences, life expectancy, overall health, and prior screening history. People over 85 should no longer get colorectal cancer screening [4].
The U.S. Preventive Services Task Force (USPSTF) recommendation states that discontinuation of screening should be considered when persons who have prior negative screening (particularly colonoscopy), reach age 75 or have < 10 years of life expectancy [5].
On the other hand, some of the other gastroenterology societies such as ASCRS [6], ACG [7] and WGO [8] guidelines have failed to demonstrate the appropriate age when to stop screening for colon cancer. A study published in JAMA (Appropriateness of colonoscopy screening) in 2014 [2] provided strong evidence to discourage the practice of screening or surveillance colonoscopy at very advanced age > 75 years.
Very recently a prospective observational large study was published in the Annals of Internal Medicine [9] which evaluated the effectiveness and safety of screening colonoscopy to prevent colorectal cancer (CRC) in persons aged 70 to 74 and those aged 75 to 79 years. Their results suggest a modest benefit of screening colonoscopy for preventing CRC in persons aged 70 to 74 years and a smaller (if any) benefit in those who are older. The risk for adverse events was low but greater among older persons.
Although recent guidelines recommended against screening for CRC after the age of 75, our study showed that in this patients' group, there is a trend toward higher proportion of high grade lesions in patients who had screening colonoscopy as well as the overall mean survival time was also more than 8 years. This should be taken into consideration while discussing screening colonoscopy in the older population who are otherwise healthy and have not had screening done in the past. Our data suggests continuing this discussion of colonoscopy screening in elderly patients with good functional status without significant comorbidities.
Although statistically significantly higher survival time was noted in patients younger than 75, older patients also had a survival time of 8.9 years. Even though a comparable percentage of HGL were found in the younger and older population, there was a trend for finding higher grade lesions in the older cohort who underwent screening colonoscopy. This should be taken into consideration when offering screening colonoscopy to the older population.
We would like to humbly thank Dr. Daniel Kruss for providing financial support for the publication of this paper through the VA CARES Research Funds. The authors have no conflict of interest. No benefit to patients was provided.
Guarantor of the article: Dr. Pallavi Shah. Dr. Shah designed the study and oversaw it to its completion; Dr. Alsayed, Dr. Ahmed, Dr. Shanshal and Dr. Desai collected and analyzed the data and prepared the manuscript draft; Dr. Molnar performed the statistical analysis. All authors read and approved the final manuscript.