Hepatitis C virus (HCV) infection is associated with increased morbidity and mortality among patients on dialysis (HD). The aims of this study were to estimate the presence of HCV and other comorbidities in the dialysis population of Elbasan city, and to compare the survival outcomes of those patients who started dialysis with haemodialysis and peritoneal dialysis.
In the present study, we have used a socio-demographic questionnaire to collect data from HD patients. We studied 108 dialysis patients in dialysis Centre for 1 year. Software SPSS version 20.0, were used to analyzed and evaluate the data. P < 0.05 was considered statistically significant.
Over of all 108 patients with dialysis, HCV infection was present in 15.7% of them. Male patients were 74% and female 26%. Age groups 50-59 and 60-69 were the most frequent among dialysis patients with 35.1% and 34.25% cases respectively. There are a significant association for age of dialysis patients and the presence of HCV for p < 0.001. Regarding the co-morbidities in dialysis patients our findings suggest that diabetes mellitus tip 2 (16/108 patients), Hypertension arterial HTA (56/108), Coronary artery disease (16/108), and Arrhythmia (12/108) were more prevalent diseases in our patients. A significant association we found for cardiovascular disease in dialysis patients and the presence of HCV p = 0.0001. Based to Kaplan-Meier and univariate Cox regression performed to estimate risk factor in haemodialysis patients, adjusted survival rates HD vs. PD patients was significantly different (hazard ratio 14.75, CI 95% [8.92-44.76]) p value < 0.0001).
In this study we have observed a significant association of HCV infection with some socio-demographic data of dialysis patients, and also with cardiovascular disease. We suggest that prospective studies should be performed to evaluate the role of HCV infection and other comorbidities in dialysis patients.
Comorbidities, Dialysis patients, HCV, Prevalence, Elbasan city
Hepatitis C virus (HCV) infection is associated with increased morbidity and mortality among patients on dialysis [1-3]. HCV virus is one of the most frequent causes of chronic viral liver disease in dialysis patients [4]. The spread of HCV in dialysis units is declining, but the prevalence of HCV in dialysis patients remains high [5]. Worldwide the prevalence of this infection in the dialysis population varies from 1% to more than 80% [6-12]. A number of risk factors have been identified for HCV infection among dialysis patients like the number of blood transfusions, duration of the dialysis treatment etc [13-15]. This high risk is due to poor infection-control measures [16,17]. Some studies have shown that the prevalence and incidence of HCV infections remain high among dialysis patients. It may be explained by transmission within dialysis centers, probably because of inadequate adherence to infection control measures [16,17]. HCV infection has been seen to play a role in pathogenesis of Diabetes Mellitus, Atherosclerosis, and Steatosis which may increase the risk of cardiovascular diseases [2,18-24] in dialysis patients. HCV-infected dialysis patients have a higher prevalence of inflammation-related metabolic and vascular diseases, leading to high rates of cardiovascular mortality in patients with end-stage renal disease. Steatosis occurs in 40%-86% of patients with chronic hepatitis C, and its frequency varies with genotype [2,18,25-27]. The aim of this study was to estimate the presence of hepatitis C virus (HCV) and co-morbidities in the dialysis patients of Elbasan dialysis unit.
This is a cohort study and was performed in dialysis unit of Elbasan city (all adults' ≥ 18-years-old) for one year. In the present study, we have analyzed 108 recorded files from dialysis patients. We have filled a questionnaire with data taken out from record files for each dialysis patient. Characteristics for socio-demographic, presence of Hepatitis C Virus (HCV) and the co-morbidities were used for each of them. The software SPSS version 20.0, were used to analyze and evaluate the data obtain in this study. Epidemiological data are presented as frequency, mean and percentage. Further statistical analysis of risk factors for HCV infection (age, number and duration on dialysis, etc) was performed by multivariate analysis and Fisher's exact test. P value < 0.05 was considered statistically significant.
Over of all 108 patients with dialysis, Hepatitis C Virus (HCV) was present in 15.74% of them (Figure 1).
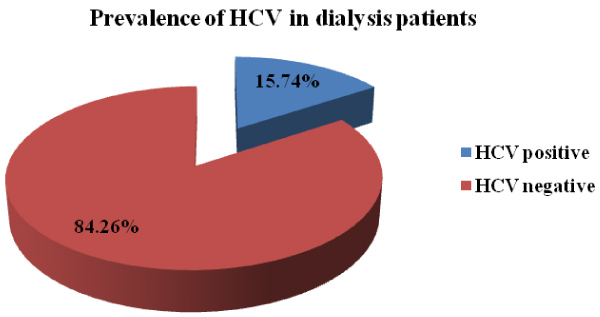 Figure 1: Prevalence of Hepatitis C in dialysis patients.
View Figure 1
Figure 1: Prevalence of Hepatitis C in dialysis patients.
View Figure 1
Regarding the gender of dialysis patients involved in our study, male patients were 74% and female 26% [28]. We found a significant association between the gender and presence of HCV infection in dialysis patients. Age groups 50-59 and 60-69 were the most frequent among dialysis patients with 35.1% and 34.25% cases respectively. The other age groups have the same percentage 10.2% of cases.
The age groups and the presence of HCV have resulted with a significant association between them for p value < 0.0001. A statistically significant association was seen also for residence area and alcohol use among patients with HCV infection. We did not find statistical association for the other risk factors and presence of HCV infection. Table 1 below represents the socio-demographic data for 108 dialysis patients.
Table 1: The socio-demographic data and presence of HCV infection in dialysis patients. View Table 1
The Table 2 below presents dialysis data of 108 patients involved in our study. In most of data obtained by record file of dialysis patients, there is a strong significance association between presences of HCV and the originated of renal disease, time of dialysis start, frequency dialysis etc. We did not find significant association for the way how patients are introduced into dialysis process.
Table 2: Dialysis data for patient involved in study. View Table 2
Presence of co-morbidities in dialysis patients came out in the most dominant part of patients. Between the Diabetes Mellitus Type 1 and Type 2, the most predominant were Diabetes Mellitus type 2, in 14.8% of all patients. The prevalence for cardiovascular diseases was 96.3% of dialysis patients. Most commonly was hypertension in 52% of all patients. HCV was most present in patients with hypertension and coronary artery disease. A significant association was seen for cardiovascular diseases and presence of HCV among dialysis patients (Table 3).
Table 3: Presence of Comorbidities among dialysis patients. View Table 3
Also, in our study we have comparing patient survival of Haemodialysis (HD) and Peritoneal Dialysis (PD). We found that the survival outcomes were differ between PD and HD patients during the month of dialysis treatment, the risk of death was significantly higher in patients initiating dialysis with PD (Figure 2).
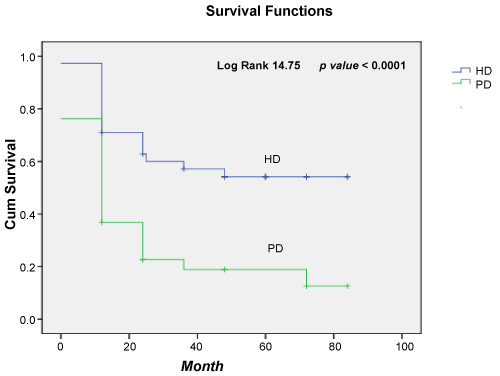 Figure 2: Survival function for HD versus PD patients.
View Figure 2
Figure 2: Survival function for HD versus PD patients.
View Figure 2
It is well known that dialysis patients are at high risk for hepatitis C infection. Interval of HCV prevalence infections among dialysis patients varies, from country to country. The prevalence rank from 3% in northern Europe more than 20% in southern Europe [29,30]. This infectious disease is a recently identified and under-recognized risk factor for patients with problems in kidneys like them in dialysis process [31]. In our study, prevalence of HCV infection among dialysis patients was present in 15.74% of them. This prevalence is lower than a study conducted by Di Lallo, et al., in Italy [13], but is to higher with another study conducted by Sandhu, et al. in Canada [32].
The HCV infection and some co-morbidity are well known as risk factors for kidney disease. The most common risk factors for kidney disease are older age, hypertension, dyslipidaemia, coronary heart disease, diabetes, and cirrhosis [32-34]. The prevalence rate of HCV infection was higher in men than female sex because male is more vulnerable to be infected with HCV than female population [35].
The finding of our study report that male was the most predominant sex (74.07%) affected by kidney disease and from HCV infection (70.6%) compared to female with significant association p < 0.0001. Older ages are a risk factor for dialysis patients [32,33], so in our study patients over 50-years-old, presented the most prevalent cases. There are a significant association for age of dialysis patients and the presence of HCV for p < 0.001. A multivariate analysis of risk factors showed a significant association with metropolitan area of residence and HCV in dialysis patients in Italy [13]. Regarding the residence area of our patients, as a risk factor for dialysis, more than half of our patients living in urban area (54.6%) and the other in rural area (45.4%) with significant association between them and presence of HCV for p value < 0.001. A significant association between alcohol use by dialysis patients and presence of HCV was seen for p value < 0.001. We didn't find significant association for the other demographic data processed by us.
Regarding the co-morbidities in dialysis patients our findings suggest that diabetes mellitus tip 2 (16/108 patients), Hypertension arterial HTA (56/108), Coronary artery disease (16/108), and Arrhythmia (12/108) were more prevalent diseases in our patients. A significant association we found for cardiovascular disease in dialysis patients and the presence of HCV p = 0.0001.
Some studies suggest that HCV infection has an adverse impact on renal function. Meyers, et al. in their study, seen that HCV infection is associated with extra-hepatic diseases, including various types of glomerulonephritis [36,37]. Also, Soma, et al. and Arase, et al. has indicated that HCV infection leads to a rapid worsening in the renal function of patients with diabetic nephropathy [38,39].
As seen in Table 2, most of data obtained by patient's files show up a strong significance between them and the presence of HCV (like, originated of renal disease, time of dialysis start time, dialysis frequency etc). We did not find significance association for the way how patients are introduced into dialysis process.
Regarding the Kaplan-Meier and univariate Cox regression performed to estimate the time of dialysis treatment as risk factor in dialysis HD vs. PD patients, adjusted survival rates was significantly different (hazard ratio 14.75, CI 95% [8.92-44.76]) p value < 0.0001. The risk of death was significantly higher in patients initiating dialysis with PD (Figure 2).
Hepatitis C Virus (HCV) is an emerging global public health issue with relevance in dialysis patients. The HCV prevalence of dialysis patients in Elbasan city remained at a moderate level of 15.74%, which might be increased among haemodialysis patients because of long-term haemodialysis and blood transfusion of patients. In this study we have observed a significant association of HCV infection with some socio-demographic data of dialysis patients, and with cardiovascular disease. We found that the survival outcomes were differ between PD and HD patients during the month of dialysis treatment, the risk of death was significantly higher in patients initiating dialysis with PD. We suggest that prospective studies should be performed to evaluate the role of HCV infection and other comorbidities in dialysis patients.
Firstly, the acknowledgements go to head of Dialysis unit in Elbasan city, for his hospitality and assistance in finalizing this paper. I want to acknowledgements co-authors for their support, assistances and contribution during the preparation of this paper. And finally, but not less important, I would like to acknowledgements all participating patients who were willing to provide the data they required. Without their helping this work would be difficult to accomplish.
Me, Brunilda Elezi, lecturer at the University Alexander Xhuvani, in Elbasan city, Albania, I declare that I don't have any conflict of interest (person of institution). All the data presented in this paper have been collected by my part and the patient's anonymity is preserved.
In our country, there is not one ethics committee that approved the study, but we are allowed to do a study after we are given permission by the head of the institution where the study is carried out.