Liver cancer is the end-stage of chronic liver diseases. The main aetiologies of chronic liver diseases CLDs are chronic hepatitis C virus (HCV) infection. The aim of this work was to evaluate diagnostic value of CD-90 in patients with chronic liver disease (CLD) Fibrosis, Liver cancer and healthy individuals. The present study included Fibrotic patients, Liver cancer patients, in addition individuals were enrolled in this study as control group. AFP and CEA were estimated in all groups. CD-90 was estimated using Flow Cytometry technique. Results from this study revealed there was high prevalence CD-90 in Liver cancer patients comparing with both Fibrosis and control group (P < 0.005). Based on our observation in this study CD-90 has diagnostic value importance in assessment of Liver cancer.
Chronic liver disease, Alpha feto protein (AFP), Carcinoembryonic antigen (CEA)
Liver diseases affect the normal functions of the liver causing decrease in its performance. Abnormalities in the liver functions, however, are usually not apparent in most individuals with chronic liver disease until the disease is rather advanced. Liver diseases could be classified into infectious (e.g. viral hepatitis), toxic (e.g. alcohol-related diseases), genetic (e.g. hemochromatosis), immune (e.g. autoimmune hepatitis primary biliary cirrhosis), and neoplastic (e.g. hepatocellular carcinoma) [1-4]. Hepatitis is inflammation of the liver. Viral infection is responsible for around half of all cases of acute hepatitis. The term is generally used to refer to the diseases caused by the hepatropic viruses including the diseases hepatitis A-E, and disease due to cytomegalovirus, Epstein-Barr virus, adenovirus, rarely herpes simplex virus and others. Only hepatitis B virus and hepatitis C virus are able to persist in the host and cause chronic hepatitis. Hepatitis C virus is also a major public health problem [5-7].
Chronic hepatitis is defined as ongoing hepatic necrosis and inflammation of the liver, often accompanied by fibrosis. Chronic hepatic injury may progress to cirrhosis and predisposes to hepatocellular carcinoma. Most commonly, it is the result of chronic viral infection. Chronic hepatic injury is a relatively common disorder with minimal symptoms but long-term risk of significant morbidity and mortality. Liver fibrosis can be classified as a wound-healing response to a variety of chronic stimuli. It is characterized by an excessive deposition of extracellular matrix (ECM) proteins which includes three large families of proteins, glycoproteins, collagens, and proteoglycans. Liver cancer is a primary malignancy of the liver and occurs predominantly in patients with underlying chronic liver disease and cirrhosis. The cell(s) of origin are believed to be the hepatic stem cells, although this remains the subject of investigation. Tumors progress with local expansion, intrahepatic spread, and distant metastases [8,9].
This study was conducted on Egyptian individuals classified into three groups as the following: 51 Fibrosis with chronic HCV infection, 30 Liver Cancer patients due to chronic HCV infection from Gastroenterology Center, Mansoura University, and 40 healthy individuals with no liver diseases. All patients recruited from Gastroenterology Center, Mansoura University, Egypt.
Six milliliters of venous blood specimens were collected from all patients and healthy control groups. Laboratory investigations included (AST, ALT) [10], total bilirubin [11], and serum albumin [12]. All were performed on Beckman CX9 autoanalyzer. AFP was determined by the ELISA technique [13]. CEA was determined by ELISA technique [14].
Human PBMC are isolated using a density gradient technique. The two most used density gradient solutions are Ficoll-Paque PLUS from (Sigma Aldrich co, USA). Flow Cytometry analysis of CD-90 from (BD Bioscience co, USA).
A computer software package (SPSS), version 16.0 was used in the analysis. For quantitative variables, mean and standard deviation. Frequency and percentage are presented for qualitative variables. Significance level (p) value was expressed as follows: p > 0.05 = Insignificant, p < 0.05 = Significant and p < 0.001 = highly significance. One-way ANOVA test was used for comparing between different groups.
Table 1, Table 2, Table 3, Table 4, Figure 1, Figure 2 and Figure 3.
Table 1: Groups characteristics in the study. View Table 1
Table 2: Laboratory biomarkers in all groups. View Table 2
Table 3: Flow cytometry of CD90 in all groups. View Table 3
Table 4: Tumor markers concentration in all groups. View Table 4
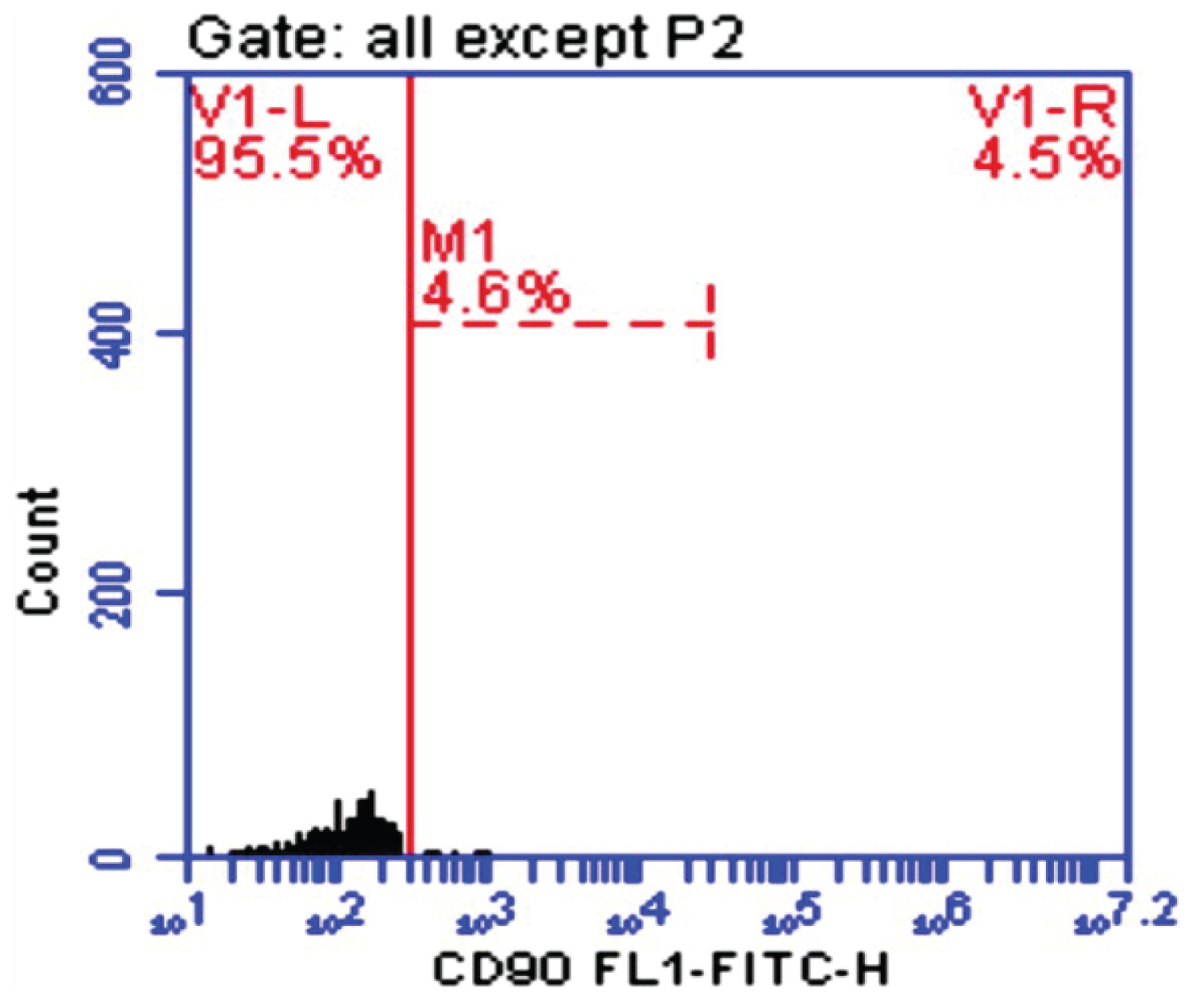 Figure1: CD-90 in control.
View Figure 1
Figure1: CD-90 in control.
View Figure 1
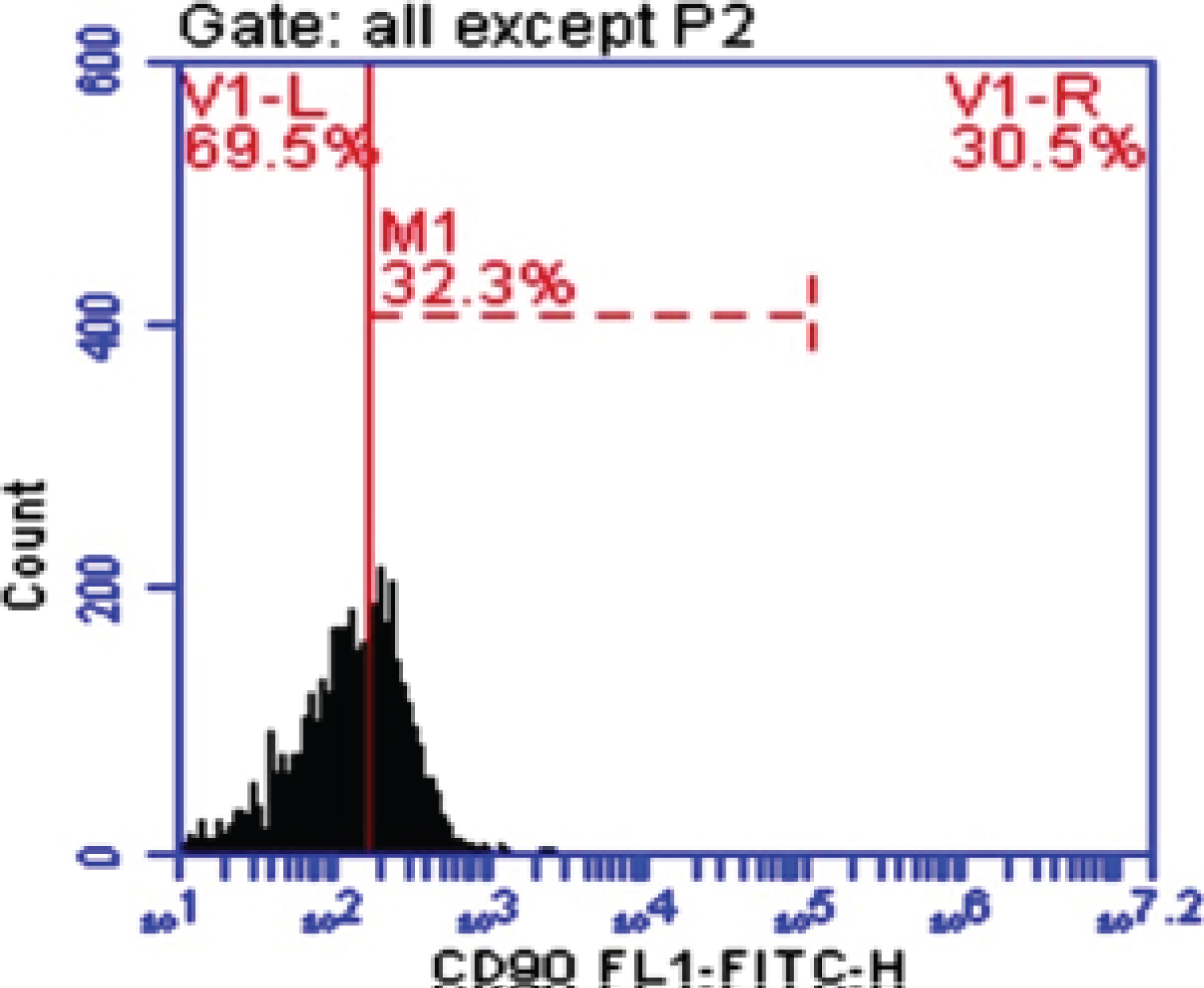 Figure 2: CD-90 in fibrosis.
View Figure 2
Figure 2: CD-90 in fibrosis.
View Figure 2
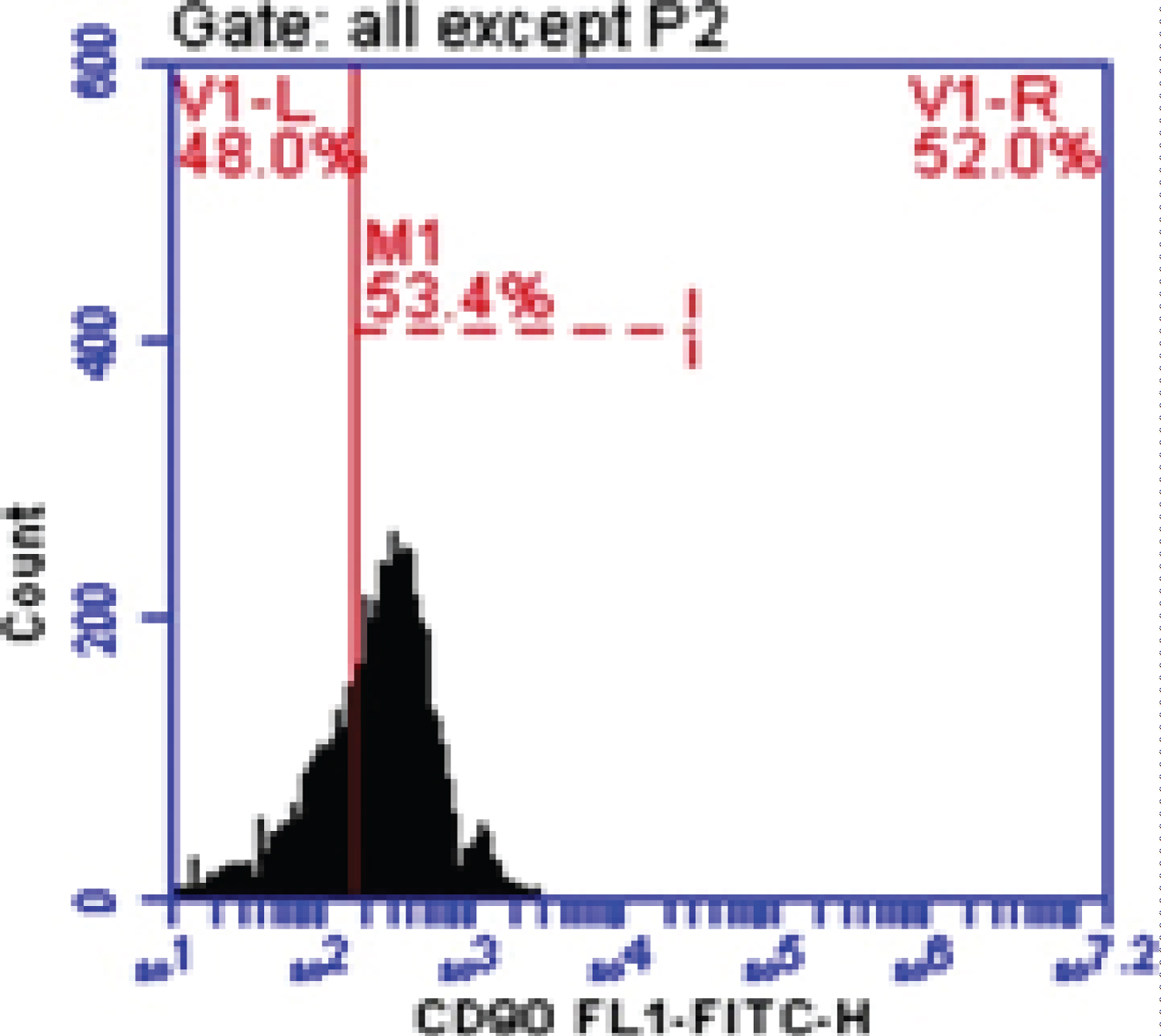 Figure 3: CD-90 in liver cancer.
View Figure 3
Figure 3: CD-90 in liver cancer.
View Figure 3
Chronic liver diseases (CLD) and its end-stages, cirrhosis and hepatocellular carcinoma (HCC), are leading causes of morbidity and mortality worldwide with enormous socioeconomic costs. The main aetiologies of CLDs are chronic hepatitis C virus (HCV) and hepatitis B virus (HBV) infections, alcohol abuse and, because of metabolic syndrome reaching epidemic proportions, an increasing prevalence of non-alcoholic steatohepatitis (NASH) [15]. In CLD, hepatocyte damage, wound healing, and tissue remodeling, resulting in fibrosis and ultimately cirrhosis, the platform on which HCC and deadly hepatic failure develop. At the cellular and molecular level, the multistep process of progressive CLD is reflected in the complex modulation of intracellular signal transduction circuits, altered cell-cell communications and, more drastically [16]. In this study our subjects were divided into 40 healthy adults with no liver diseases as a control group 28 males (70%) and 12 females (30%), and their age ranged from 20-51 years with a mean of 30.8 ± 7.57 years. 51 Fibrosis (F2-F4) patients, 42 males (82.4%) and 9 females (17.6%), their age ranged from 43-87 years with a mean of 59.9 ± 10.1 years. 30 HCC patients, 23 males (76.7%) and 7 females (23.3%), and their age ranged from 43-74 years with a mean of 57 ± 8.81 years. This finding was close to that of Hussein, et al. [17] and Hernandez-Castillo, et al. [18] and Massoud, et al. [19] who reported that the mean ages among Liver cancer cases were 53.7 ± 10.1, 57.4 ± 8.7, and 55.2 ± 8 years, respectively.
In the present study ALT was increased in Fibrosis and HCC patients significantly compared with Healthy control group Mean ± SD. were 59.9 ± 36.7, 56.3 ± 35.7 and 12.15 ± 2.6; respectively (P < 0.005). Also, AST was increased in both Fibrosis and Liver cancer patients comparing with Healthy control group Mean ± SD. were 73.2 ± 35.6, 70.1 ± 28.3 and 12.4 ± 2.7; respectively (p < 0.005). Serum bilirubin was increased significantly both HCC and Fibrosis patients comparing with Health control group with values 7.4 ± 11.0, 4.1 ± 6.3 and 0.3 ± 0.18; respectively (p < 0.005). In our study albumin was decreased significantly in both Liver cancer and Fibrosis patients comparing with Healthy control group with values 2.91 ± 0.25, 3.2 ± 0.15 and 4.3 ± 0.43; respectively (p < 0.005). The elevated aminotransferase value in Liver cancer reflects damage to adjacent hepatocytes as a direct result of tumor growth or damage to more remote liver cells caused by interference with their blood supply or venous drainage. It may also be due to continuing liver cell necrosis in those with concomitant active cirrhosis or chronic active hepatitis [20]. In our study, AFP was significantly higher in Liver Cancer patients compared to Fibrosis group.
CD90 is a 25-37 kDa glycophosphatidylinositol (GPI)-anchored protein expressed in many cells such as T-cells, thymocytes, neurons, endothelial cells and fibroblast. CD90 operates as an important regulator of cell to cell and cell to matrix interaction, apoptosis, adhesion, migration, cancer and fibrosis [21-23]. In diseased liver, CD90 was expressed in hepatic stem cells, hepatic fibroblasts, myofibroblasts, and tumor stroma (CAFs), and small percentage of CSC. The origin of the myofibroblasts and CAFs might be derived from the epithelial mesenchymal transition (EMT), resident fibroblasts, or bone marrow derived stem cells. It has been demonstrated that multipotent adult stem cells with CAFs properties was found not only in HCC, but also in cirrhotic liver, supporting the evidence that the CAFs could be originated from resident progenitor cells [24]. In our study blood peripheral mononuclear cells CD90 (M1%) was increased significantly in HCC and Fibrotic patients comparing with Healthy control group with Mean ± SD. values 46.29 ± 4.0, 39.66 ± 5.49 and 6.13 ± 2.84 respectively (P < 0.005). This is in accordance with some previous studies on HCC cell lines and human samples, where CD90 was highly expressed in malignant hepatocytes and the presence of CD90+/CD44+ cells contributed to an aggressive phenotype with more frequent metastatic lesions in the lung [25]. Our data was in agree with Bahnassy, et al. [26] who found that CD90 was significantly higher in the blood of HCC patients compared to those in the CH and control groups (P < 0.001).
Based on our observation in this study CD-90 has diagnostic value in assessment of patients with chronic hepatitis-c virus and hepatocellular carcinoma.
The author would like to thank Dubai Medical College and Gastroenterology Center, Mansoura University for this work.