Obesity, defined as a body mass index (BMI) ≥ 30 kg/ m2, is a global public health issue. Obesity is associated with comorbidities such as diabetes, hypertension, dyslipidemia, obstructive sleep apnea, and non-alcoholic fatty liver disease and is known to significantly impair quality of life and reduce the life expectancy [1]. Worldwide obesity has nearly tripled since 1975 and in 2016, an estimated 650 million adults (13% of the world's adult population) were obese, and by year 2030, it is estimated that 19.7% of the world's population (1.12 billion individuals) will be obese [2,3].
Treatment options for obesity include lifestyle interventions, pharmacotherapy, and bariatric surgery. The efficacy of currently available anti-obesity pharmacotherapy is far less than it was expected (estimate weight loss of 3-7% compared with placebo) [4,5]. On the other hand, bariatric surgery, although very effective, is associated with significant morbidity (3-20%) and substantial cost and is available for only fewer patients [6-8]. Bariatric endoscopic procedures are at the horizon and can be a bridge between bariatric surgery and pharmacotherapy, by effectively replicating the anatomic and physiological changes of the traditional weight-loss surgeries, while at the same time being less invasive, more cost effective and reversible [9-11]. Bariatric endoscopy therapy is categorized in mainly three different types: Restrictive, Malabsorptive and others [12]. Restrictive procedures act by decreasing the gastric volume by space-occupying devices and/or by suturing or stapling techniques, whereas malabsorptive procedures tend to create malabsorption by preventing the food contact with the duodenum and proximal jejunum [12].
Malabsorptive procedure mimics Roux-n-Y gastric bypass (RYGB) surgery. RYGB exhibits significant hormonal changes after surgery which results in acute and immediate glycemic control via an anti-diabetic weight-independent mechanism, even without significant weight loss after surgery. Therefore, malabsorptive procedure can be considered metabolic procedure because it will help in reducing weight as well as type 2 diabetes mellitus (T2DM). In this review article, we aim to provide an overview on the role of small intestine on obesity and metabolic syndrome and different newer Endo Bariatric procedure focusing on small intestine.
It is very important to understand the role of small intestine in the pathophysiology of obesity in order to understand the effective treatment or endoscopic procedures. Recent insights have revealed the critical physiologic and pathophysiologic role of the small bowel in metabolic homeostasis and its potential role as a driver of obesity, insulin resistance, and subsequent T2DM [13]. Although the other parts of the small bowel cannot be ignored when describing the potential mechanisms involved in the development of metabolic diseases and T2DM, the excellent endoscopic accessibility of the duodenum makes it a prime target for disease-modifying intervention [13].
Intestinal mucosal maladaptation and bacterial translocation have been linked to the endotoxemia in response to caloric overexposure [8]. Chronic endotoxemia induces low-grade systemic inflammation, which is associated with increases in fasting plasma glucose, insulin, visceral adipose tissue, and bodyweight, all leading to the phenotypic features of the metabolic syndrome, obesity, and T2DM [14]. Additionally, a high-fat diet also stimulates the proliferation of duodenal endocrine K cells, producing glucose-dependent insulinotropic polypeptide, which is thought to induce insulin hypersecretion [15]. Upon duodenal-jejunal bypass (Roux-en-Y surgery), epithelial proliferation and tight junction expression are increased, which subsequently leads to decreased intestinal permeability and decreased endotoxemia in patients with obesity and T2DM [16-18].
Obesity is associated with a reduction in the abundance of Bacteroidetes and a proportional increase in Proteobacteria, which could all be an epiphenomenon, but could also be the expression of a microbiota-related mechanism in the pathophysiology of obesity and T2DM in some individuals [13,19]. RYGB surgery restores the gut microbiome toward a healthy composition, which includes an increased diversity in flora and that may be another reason of improvement in obesity and T2DM [20].
Glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP) are incretin hormones which are released by endocrine L cells and K cells throughout the small intestine in response to nutrients in the lumen [13]. GLP-1 stimulates insulin secretion from pancreatic b-cells, inhibits glucagon secretion from pancreatic a-cells, and decreases gastrointestinal motility, appetite, and food intake [13]. The incretin effect is decreased in patients with T2DM, especially from L cells in the duodenum and jejunum [21]. GLP-1 when given exogenously reduces bodyweight and hyperglycemia in patients with T2DM. The 'hindgut hypothesis' of RYGB surgery states that surgical re-routing of nutrients to the distal part of the small intestine results in increased secretion and concomitant glucose-lowering effects of GLP-1 [22]. The "foregut hypothesis" proposes that the exclusion of the duodenum and proximal jejunum from the transit of nutrients may prevent the secretion of a putative signal that promotes insulin resistance and T2DM, suggesting that a yet unidentified inhibitory product from the proximal bowel causes metabolic changes (anti-incretin) [22] (Figure 1).
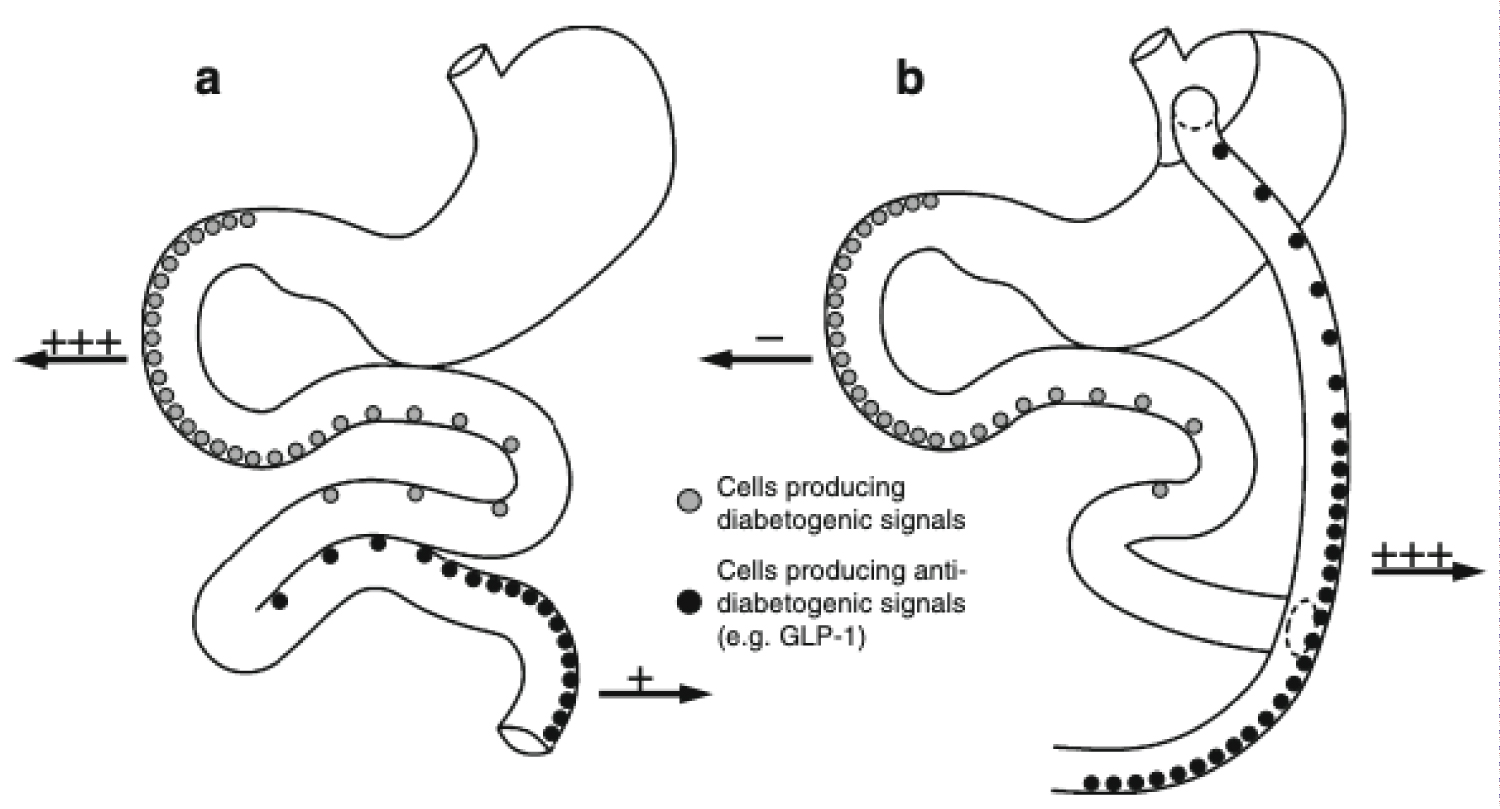 Figure 1: (a) The normal anatomy of the stomach, duodenum and jejunum shown schematically. The "foregut hypothesis" proposes that when the foregut of susceptible individuals is overstimulated with nutrient it releases a diabetogenic signal resulting in the development of type 2 diabetes; (b) Anatomical changes produced by RYGB are indicated. These include division of the stomach into a small upper pouch and a much larger, lower 'remnant' pouch, division of the small bowel about 45 cm below the lower stomach outlet, and re-arrangement into a Y configuration, enabling outflow of food from the small upper stomach pouch, via a "Roux limb". The Y intersection is formed 80-150 cm from the anastomosis between the small upper gastric pouch and the small bowel. The foregut hypothesis that following RYGB, diabetogenic signaling is avoided explaining the 'acute' RYGB-induced resolution of type 2 diabetes. The elaboration of the foregut hypothesis presented in the present paper suggests that part of the 'foregut' diabetogenic signals consists of glucagon itself and/or glucagonotropic signaling (e.g. by the hormone GIP) (indicated by '+++' in (a)), which, in susceptible individuals, during normal anatomical conditions, would result in increased hepatic glucose output and subsequent hyperglycemia. Following RYGB, these signals would be circumvented (indicated by '--' in (b)) and therefore, in combination with increased anti-diabetogenic signaling (i.e. GLP-1 secretion; indicated by '+++' in (b)) from the hindgut, result in amelioration of the diabetic state [22]. View Figure 1
Figure 1: (a) The normal anatomy of the stomach, duodenum and jejunum shown schematically. The "foregut hypothesis" proposes that when the foregut of susceptible individuals is overstimulated with nutrient it releases a diabetogenic signal resulting in the development of type 2 diabetes; (b) Anatomical changes produced by RYGB are indicated. These include division of the stomach into a small upper pouch and a much larger, lower 'remnant' pouch, division of the small bowel about 45 cm below the lower stomach outlet, and re-arrangement into a Y configuration, enabling outflow of food from the small upper stomach pouch, via a "Roux limb". The Y intersection is formed 80-150 cm from the anastomosis between the small upper gastric pouch and the small bowel. The foregut hypothesis that following RYGB, diabetogenic signaling is avoided explaining the 'acute' RYGB-induced resolution of type 2 diabetes. The elaboration of the foregut hypothesis presented in the present paper suggests that part of the 'foregut' diabetogenic signals consists of glucagon itself and/or glucagonotropic signaling (e.g. by the hormone GIP) (indicated by '+++' in (a)), which, in susceptible individuals, during normal anatomical conditions, would result in increased hepatic glucose output and subsequent hyperglycemia. Following RYGB, these signals would be circumvented (indicated by '--' in (b)) and therefore, in combination with increased anti-diabetogenic signaling (i.e. GLP-1 secretion; indicated by '+++' in (b)) from the hindgut, result in amelioration of the diabetic state [22]. View Figure 1
Bile acids are a key stimulus for the farnesoid X receptor in the liver [23,24] affecting hepatic metabolism, and G-protein-coupled bile acid-activated receptors (TGR5) of the enteroendocrine L-cells [25,26] and promoting the release of incretin. Therefore, bile acids play an important role in glucose homoeostasis. Post-operative increases in circulating bile acids have been suggested to contribute to the metabolic benefits of bariatric surgery; however, their mechanisms remain undefined.
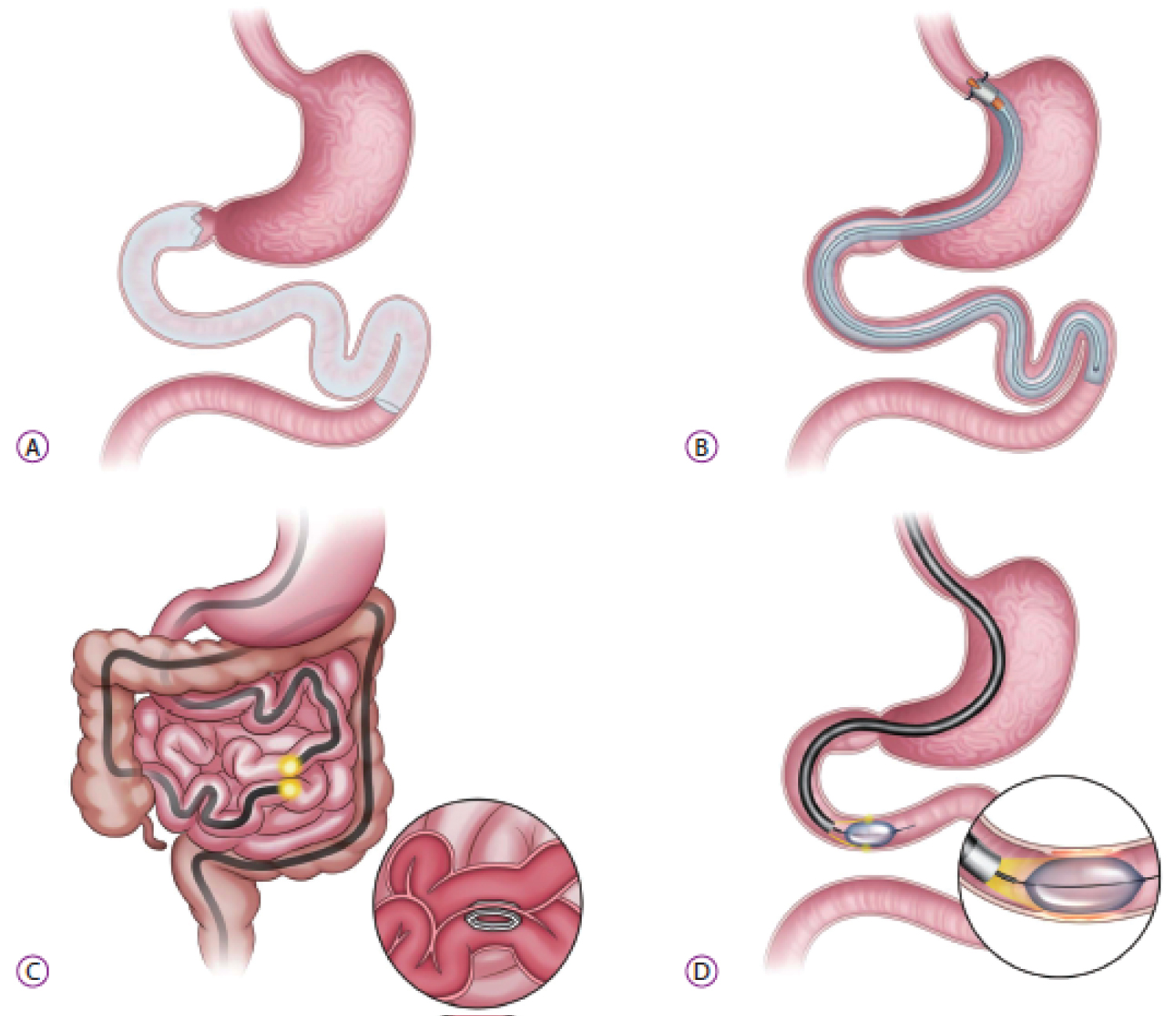 Figure 2: Small bowel endoscopic bariatric therapies (A) Duodenaljejunal bypass liner; (B) Gastroduodenojejunal bypass sleeve; (C) Incisionless anastomosis system; (D) Duodenal mucosal resurfacing [28].
View Figure 2
Figure 2: Small bowel endoscopic bariatric therapies (A) Duodenaljejunal bypass liner; (B) Gastroduodenojejunal bypass sleeve; (C) Incisionless anastomosis system; (D) Duodenal mucosal resurfacing [28].
View Figure 2
The endoscopic duodenal jejunal bypass sleeve (DJBS), now marketed as the EndoBarrier Gastrointestinal Liner (GI Dynamics Inc., Lexington, MN, USA), was first reported by Rodriguez Grunert, et al. [27] in 2008. It is a 60 cm long, highly flexible, Teflon fluoropolymer which is deployed under endoscopic and fluoroscopic guidance (Figure 2A). It has a self-expanding nitinol anchor in the duodenal bulb that allows the barbs to fixate within the gastrointestinal tract, hence decreasing the risk of migration. This sleeve extends from duodenal bulb to the proximal jejunum, allowing chyme to pass directly from the stomach into the jejunum, making an intestinal bypass/biliopancreatic diversion without the need for stapling or anastomosis. Pancreatic enzymes and bile flow down between the liner and the intestinal wall, mixing with nutrients at the jejunum [28]. The sleeve stays in the place from 3 to 12 months and then removed endoscopically [28]. Both the exclusion of the duodenal-jejunal nutrient flow and rapid delivery of undigested nutrients and bile acid to the distal small intestine are thought to play roles in the weight loss and improvement of glucose metabolism [22]. (Foregut hypothesis and hindgut hypothesis as described above).
Although this technology has yet to be approved by FDA within the United States, there have been multiple clinical trials which have shown promise with this device with significant weight loss and improvements in several metabolic parameters [29-33]. In a large meta-analysis by ASGE taskforce published in 2015, endobarrier was associated with 35.3% excessive weight loss (EWL) at 12 months (95% CI, 24.6%-46.1%) and additional -1% improvement in HbA1C (95% CI, -1.67 to -0.4; P 0.001) when compared with a sham or control diabetic group [34]. Another meta-analysis by Rhode, et al. [35] in 2016, showed that DJBS resulted in additional 12.6% EWL (95% CI, 9.0%-16.2%, I2 = 24%) and additional -0.8% improvement in HbA1C (95% CI, -1.8 to 0.3, I2 = 53%) as compared with diet modification in patients with obesity and T2DM. A retrospective study of diabetic patient's undergone liver elastography for NAFLD has evidenced a significant reduction of liver stiffness and controlled attenuation parameter (CAP) in patients treated with DJBS for 1 year [36]. A recent meta-analysis by Jirapinyo, et al. [37] in 2018 showed total body weight loss (TBWL) of 18.9% [95% CI, 7.2%-30.6%], and EWL of 36.9% [95% CI, 29.2%-44.6%] at 9 months and a decrease in HbA1c level of 1.3% (95% CI, 1.0%-1.6%) relative to control subjects. The amount of weight loss remained significant at 1 year post-explantation [37] but a recent long term follow- up study revealed that the weight reduction of initial DJBS treatment seems to be diminished after 4 years of follow up [38].
Adverse events reported with the DJBS include abdominal pain, nausea, and vomiting [35]. Almost all patients with DJBS have been reported to experience mild-to-moderate adverse events, which can be improved with conservative management [35]. Associated serious adverse events include sleeve migration (4.9%), gastrointestinal bleeding (3.9%), sleeve obstruction (3.4%), liver abscess (0.1%), cholangitis (0.1%), acute cholecystitis (0.1%), and esophageal perforation (0.1%) [34]. Early device removal has been required in up to 38% of patients, due to bleeding, migration, obstruction, or abdominal pain [34,35,37]. No case of procedure-related mortality has been reported. However, one multicenter, randomized, sham-controlled United States pivotal trial named ENDOtrial was prematurely abandoned after enrolment of 325/500 patients due to a relatively high incidence of hepatic abscess formation (3.5%) [39]. For safety reasons, the device did not receive FDA approval, whereas CE mark was achieved in 2009, then withdrawn in 2017 [40]. Due to the results discussed above, the current system fell out of favor but paved the way for second generation DJBS with atraumatic anchoring and retrieval systems, which are being currently studied in clinical human trials.
Gastroduodenal-DJBS (GDJBS; ValenTx Endo Bypass System, Inc., Hopkins, MN, USA) is another bariatric endoscopic malabsorptive procedure (Not FDA approved yet). It is a 120-cm-long fluoropolymer sleeve designed to be anchored to the gastroesophageal junction and then it extends from the stomach to the jejunum, hence food passes directly from the esophagus into the small bowel avoiding absorption of nutrients in the stomach, duodenum, and jejunum [28] (Figure 2B). The implantation procedure is both endoscopic and laparoscopic, with laparoscopy allowing for external visualization to ensure transmural anchor placement at the gastroesophageal junction. The device can be removed endoscopically through endoscopic ligation of the anchoring sutures [28]. In a small cohort of 24 patients, Sandler and colleagues found that the device was successfully delivered in 22 (92%) patients, with five patients (23%) requiring early removal of the device because of odynophagia [41]. In remaining 17 patients, the device resulted in 39.7% EWL (range, 27%-64%) at 3 months, and 7 patients with T2DM had normal blood glucose levels throughout the trial [41]. In other study, the same authors published the results of GDJBS at 12 months on 13 patients [42]. One patient was excluded because of inflammation of gastroesophageal junction during endoscopic evaluation and two patients required early explantation (within the first 4 weeks) of the device due to intolerance. Of remaining 10 patients, six patients had fully attached functional devices till the end of follow-up period and GDJBS achieved a mean EWL of 54% at 12 months without any major side effects. Four patients had partial cuff detachment and the mean EWL was lower than that of the patients with full attachment. Also, in the subgroup, obesity-related comorbidities (diabetes, hypertension, dyslipidemia) showed improvement during the trial [42].
Duodenal mucosal resurfacing (Fractyl Laboratories, Inc., Lexington, KY, USA) (not FDA approved, received CE mark) is a novel, minimally invasive endoscopic procedure that involves circumferential hydrothermal ablation of the duodenal mucosa through a 2-cm balloon filled with heated water under direct endoscopic visualization [28] (Figure 2D). A polyethylene terephthalate balloon catheter is introduced into the duodenum, and circumferential mucosal lifting is performed along the length of the postpapillary duodenum. After that, a 2.0-cm-long balloon on the catheter is inflated with heated water at 90 C for circumferential ablation of the duodenal mucosa for 10 seconds in each application [28]. Endoscopist should only start ablation after the ampulla of vater to prevent complication.
It is hypothesized that DMR results in the destruction of diseased duodenal mucosa and subsequent regeneration and resetting of the duodenal mucosa [43]. This approach has shown to achieve only mild effects as a bariatric treatment but positive outcomes in terms of glycemic control in patients with T2DM [44]. In a first proof of concept study by Rajagopalan and colleagues [45], 39 patients with T2DM underwent DMR procedure, with consequent improvement in HbA1C (mean of 1.2% ± 0.3%) and modest weight loss of 3% TBWL at 6-month follow-up. Full-length DMR had greater effect on glycemic control, but the difference was not statistically significant than short-length DMR [45]. The recently published multi-center, open-label study by Baar and colleagues [46] has also evaluated safety and efficacy of DMR in 48 patients with T2DM (HbA1c 7.5-10.0%) on stable oral glucose-lowering medication. Twenty-four weeks post-procedure, HbA1c has significantly decreased (-0.9% ± 0.2%), while weight was only modestly reduced (-2.5 ± 0.6 kg). Effects were sustained at 12 months [46]. Not only DMR improves the glycemic control, but it helps with NAFLD parameter as evident by a recent cohort study of NAFLD diabetic patients in which DMR led to a reduction of ALT and FIB-4 at 6-month follow up [47].
At least one AE related to DMR was reported by 52% of participants; however, majority of them were mild [46]. Duodenal stenosis was noted in 3 patients (7.6%) that was treated with endoscopic balloon dilation [45]. We might presume that adequate submucosal lift and avoidance of overlapping ablations might reduce the risk of this complication, but further data are needed.
Ongoing clinical trials are aimed to clarify the effects of DMR in patients with type-2 diabetes and other chronic diseases such as polycystic ovary syndrome [48].
The incisionless magnetic anastomosis system (IMAS; GI Windows, West Bridgewater, MA, USA) (Not FDA approved) is used for the formation of a dual-path enteral bypass permitting ingested nutrients and digestive fluids to go along the native anatomy and the jejuno-ileal anastomosis [12] (Figure 2C). This system requires simultaneous enteroscopy and colonoscopy, whereby the self-assembling magnets are deployed from the working channel of each endoscope into the jejunum and in the ileum [28]. Once deployed and coupled, the octagonally shaped magnets create a large-bore compression anastomosis that allows partially digested contents to pass rapidly to the distal ileum. The coupled magnets pass spontaneously through the stool; therefore, the dual-path enteral bypass system does not require further endoscopy to remove the device [28]. This partial jejunal diversion is unlike a jejuno-ileal bypass surgical procedure that creates a blind defunctionalized segment of small intestine, which may result in a number of serious AEs [12]. Nevertheless, its metabolic effects are favorable due to gut hormone modulation similar to those seen with biliary pancreatic diversion with duodenal switch or ileal transposition surgery [12].
In the first pilot study of 10 morbidly obese patients with a mean body mass index of 41 kg/m2 demonstrated the technical feasibility and durable patency of dual-pass enteral bypass [43]. The total procedure time was 115 min. The average TBWL was 14.6% and 40.2% EWL at 12 months. A significant reduction in HbA1c level was observed in all diabetic (-1.9%) and prediabetic (-1.0%) patients, while reducing or eliminating the use of diabetes medications [49]. No serious AEs occurred, but most patients had transient nausea and diarrhea that resolved without sequel [49].
Small intestine is a very important part of the gastrointestinal tract where multiple external factors (eg, nutrients, microbiome) and internal factors (eg, bile acids, mucosal sensing, enteroendocrine function) contribute to metabolic (dys) regulation. The accessibility of the duodenum to gastroenterologists renders it an attractive target for new experimental bariatric and metabolic procedures aimed at finding novel therapeutic leads. Various experimental small bowel EBTs have bright future because they have produced promising results in weight loss and metabolic parameters, with reasonable safety profiles. Small Bowel EBTs are more effective than lifestyle modification and are less invasive and thus safer than bariatric surgery, however its long-term efficacy is less durable than bariatric surgery. Further research is required to improve the technicality of the existing small bowel EBT and establish its long-term effects; and perhaps develop new small bowel EBT for even better safety and efficacy in near future.