Lactobacilli are important bacteria among intestinal microflora. They can prevent intestinal colonization by pathogenic bacteria, and used as probiotics.
The aim of this study was to investigate the rate Lactobacillus colonization in the fecal of healthy breast-fed infants.
Fecal samples were collected from 90 healthy breast-fed infants under 18 months. Lactobacilli were identified by phenotypic and physiological methods. Carbohydrate fermentation patterns were determined using a miniplate method.
Out of 90 infants' stool samples, 72 (80%) samples were positive for lactobacillus. In total 238 isolates were identified as lactobacillus. The dominated species were L. casei (55%), L. plantarum (20.6%) and L. reuteri (8.8%) respectively.
Since some lactobacilli strains have health-promoting effects on infant and are use as probiotics, therefore further investigation on probiotic potential of identified species may help in finding new probiotics.
Lactobacillus, Identification, Infant, Fecal, Microflora
The gastrointestinal microflora of human contains complex community of microbial species including approximately 400 different microbial species, mostly bacteria [1]. Lactic acid bacteria and bifidobacteria are important group among intestinal microflora, since they have health-promoting benefits, such as the prevention of intestinal colonization by pathogens [2,3]. They are commonly used as probiotics, with effects especially against acute diarrhoea in childhood [4,5].
Lactobacilli species are gram positive, catalase negative, non-spore-forming and aerotolerant or anaerobic bacteria. They are found in dairy, grain, meat and fish products, beer, fruits, pickled vegetables, silage, sourdough, soil, as well as mouth, intestinal tract, and vagina of humans and many animals as normal flora [6,7]. Before the birth gastrointestinal tract of fetus is sterile, but microbial colonization immediately begins after the birth [8,9]. Several factors such as mode of delivery, preterm birth, antimicrobial agents, hygienic conditions, diet (breast or formula feed) and etc., affect microbial presence and succession in gastrointestinal tract of infants [1,10].
The rate of morbidity and mortality in breast-fed infants is lower than formula-fed infants. In several studies especially in poor hygienic status countries, an inhibiting effect of breast-fed on enteritis has been shown [8]. Some studies indicated that bifidobacteria and lactobacilli are dominated microflora in the breast-fed infants, and in formula-fed infants mostly were bacteroides, clostridia and Enterobacteriaceae [11]. However there is a controversy about the extent of lactobacilli colonies the intestines of newborn infants and small children [4].
There are only limited studies about characterization of lactobacilli species in stool microflora of healthy, breast-fed infants. The aim of this study was to investigate the rate of lactobacilli in fecal microbial of Iranian breast-fed infants.
Fecal samples were collected from 90 healthy breast-fed infants under than 18 months, < 1 month (24 samples), 1-3 (11), 3-6 (20), 6-9 (11), 9-12 (13), and 12-18 (11). About one gram of each sample was immediately cultured in 50 ml MRS broth (Scharlau Chemie S. A, Barcelona, Spain) and incubated at 37 ℃ for 48-72 h under anaerobic condition. Then, samples were sub-cultured on MRS agar (Scharlau Chemie S. A, Barcelona, Spain) plates and incubated at 37 ℃ for 48 under anaerobic condition. Three to four suspicious colonies of each plate were tested. Gram positive catalase negative rods regarded as lactobacilli and kept at -20 ℃ in MRS broth containing 20% glycerol. This study was approved by the Research Ethics Committee of Tehran University of Medical Sciences (TUMS), and informed parental consent was obtained.
The fermentation of carbohydrates was determined according to Kandler (1986) using the miniplate method described by Oberhofer (1983) [12,13]. All isolates were investigated for the following 14 sugars (Merck): L-arabinose, D-xylose, galactose, D-glucose, mannitol, mannose, sorbitol, cellobiose, maltose, lactose, melibiose, saccharose, trehalose and raffinose. The basal medium (MRS without glucose and meat extract, but with 0.005% bromcresol purple) contain a final concentration of 0.5% (w/v) of each sugar was used. To ensure anaerobic conditions, two drops of sterile liquid paraffine (Merck) were placed in each well of miniplate (Nunc) after inoculation. Miniplates were incubated at 30 ℃ and were examined after 2 and 5 days [14,15].
Hydrolysis of arginine was tested in MRS broth without glucose and meat extract but containing 0.3% arginine and 0.2% sodium citrate replacing ammonium citrate. Ammonia was detected after 3 days using Nessler's reagent (Merk) [13]. Gas (CO2) production from glucose was observed in MRS broth containing inverted Durham tubes after 5 days at 30 ℃ [16]. Growth at different temperatures was observed in MRS broth after incubation for 3 days at 15 ℃ or 45 ℃. Identification was performed according to Bergey's manual of systematic bacteriology [17].
Out of 90 infants' stool samples, 72 (80%) samples were positive for lactobacillus, and 238 isolates were identified as lactobacillus. Physiological and biochemical characteristics of the lactobacilli isolates are shown in Table 1. Isolates were classified to three physiological groups: 1) The obligate homo-fermentative lactobacilli, 2) The facultative homofermentative lactobacilli and 3) The obligate hetero-fermentative lactobacilli.
Table 1: Physiological and biochemical characteristics of identified species. View Table 1
The most identified species from fecal microflora of infants were 131 L. casei (55%), 49 L. plantarum (20.6%) and 21 L. reuteri (8.8%), followed by L. fermentum (6%), L. intestinalis (3%), L. curvatus (2.5%), L. johnsonii (1.26%), L. paracasei (1.26%), L .brevis (0.84%) and L. acidophilus (0.84%) respectively Figure 1.
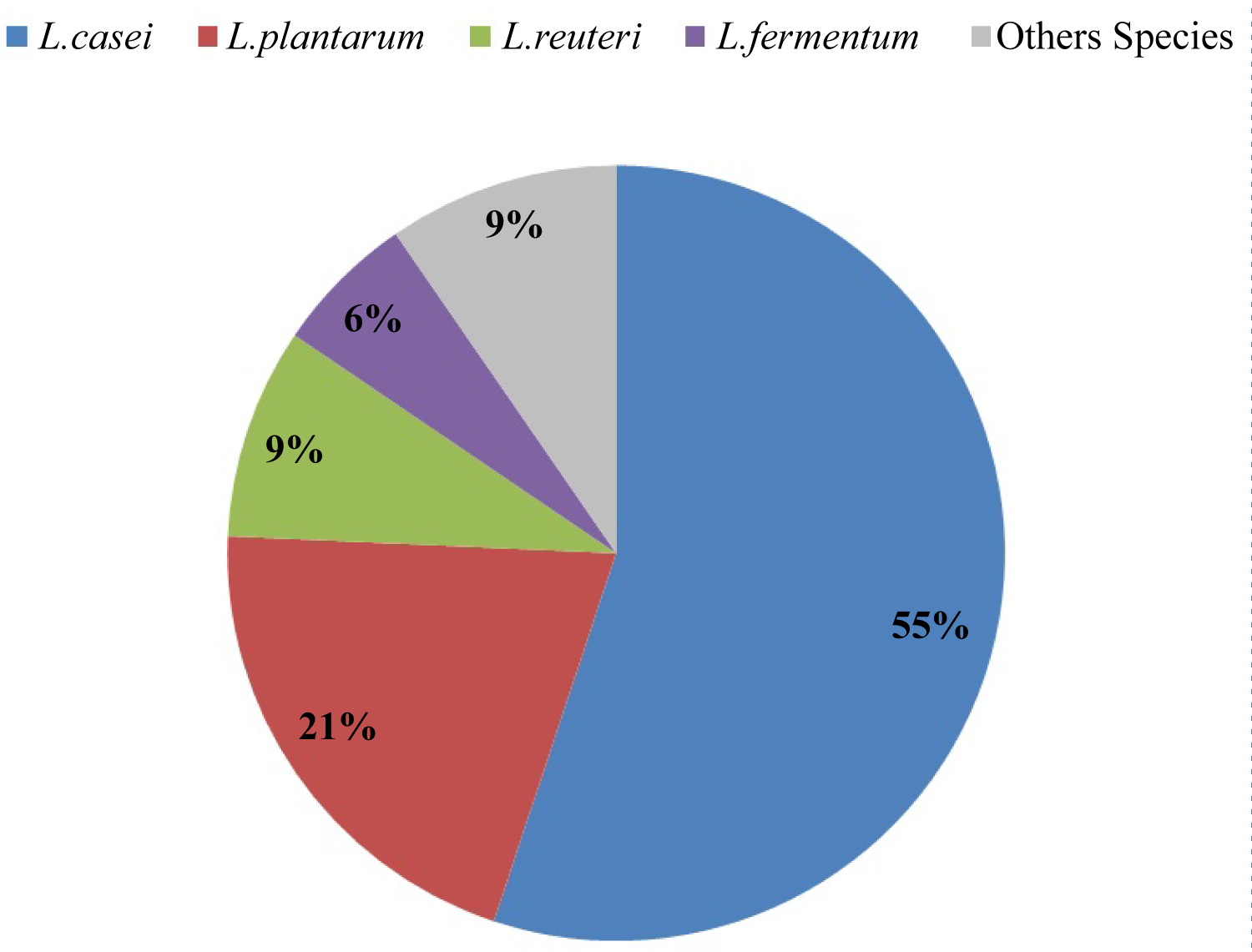 Figure 1: Frequency of isolated Lactobacilli species.
View Figure 1
Figure 1: Frequency of isolated Lactobacilli species.
View Figure 1
In general 18 (75%) stool samples of infants under I month were positive for lactotobacilli as follow: L. casei (22 isolates), L. plantarum (14), L. reuteri (14), L. fermentum (6), L. intestinalis (1), L. curvatus (1), L. johnsonii (1) and L. paracasei respectively (1). The frequency of four commonly lactobacillus species in different age groups of infants are shown in Figure 2.
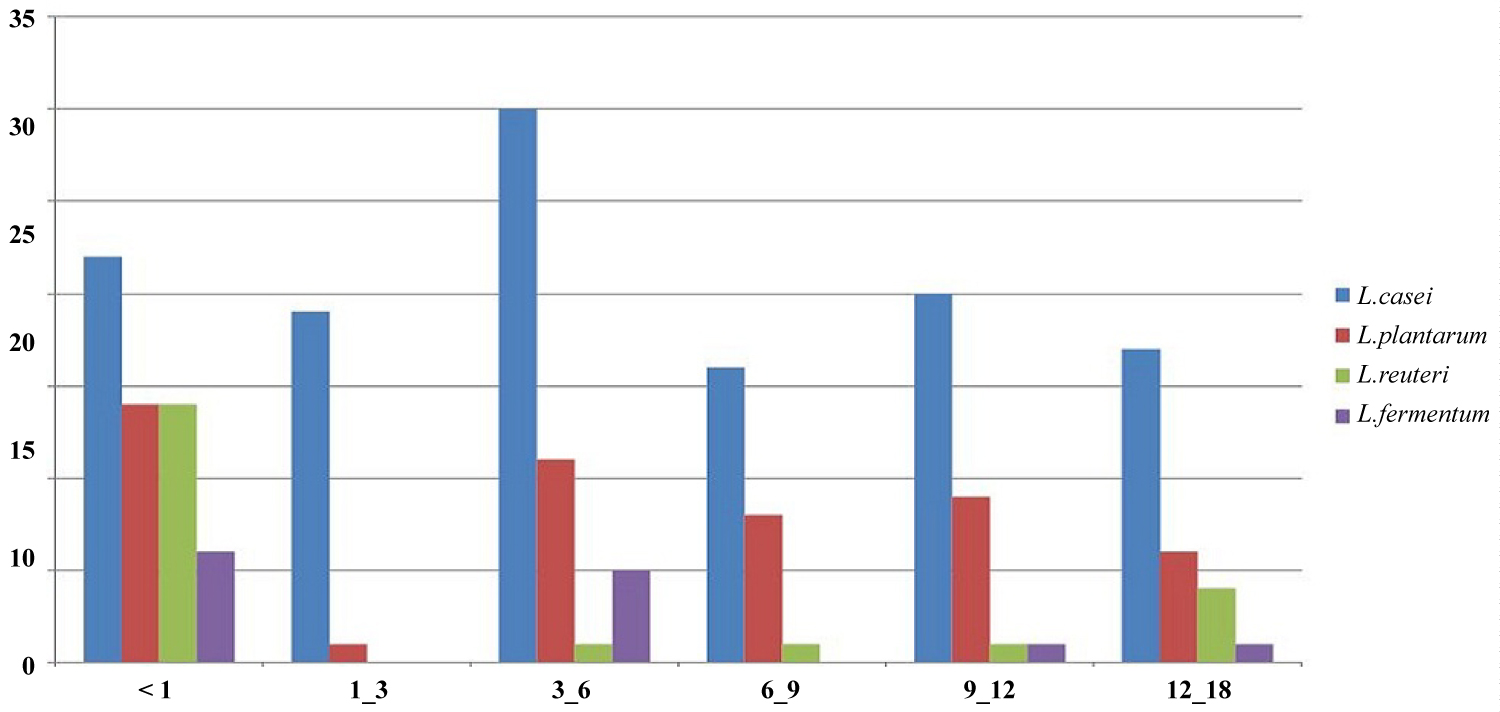 Figure 2: Frequency of four commonly Lactobacillus species in different age groups of infants.
View Figure 2
Figure 2: Frequency of four commonly Lactobacillus species in different age groups of infants.
View Figure 2
Eight (72%) of stool samples of infants between 1 and 3 months were positive for lactobacilli, and identified species from this group were L. casei (19 isolates), L. plantarum (1), L. intestinalis (1), L. curvatus (1), L. brevis (1) and L. paracasei (1).
In infants between 3-6 months, 16 (80%) stool samples were positive and the identified species were L. casei (30 isolates), L. plantarum (11), L. fermentum (5), L. intestinalis (5), L. curvatus (2) and L. reuteri (1).
In infants between 6-9 months, 9 (82%) stool samples were positive and the identified species were L. casei (16 isolates), L. plantarum (8), L. acidoplilus (1) and L. reuteri (1).
At the age between 9-12 months, 11 (84%) stool samples were positive and identified species from this group were L. casei (20 isolates), L. plantarum (9), L. curvatus (2), L. reuteri (1), L. fermentum (1), L. johnsonii (1) and L. paracasei (1).
And finally in age of between 12 to 18 months 10 (91%) stool samples were positive for lactotobacilli and identified species were L. casei (17 isolates), L. plantarum (6), L. reuteri (4), L. fermentum (1), L. johnsonii (1), L. acidoplilus (1) and L. brevis (1).
Few studies have examined lactobacilli diversity at the species level in fecal microflora of infants and children. The human milk has some ingredients and properties such as low content of iron and phosphorus, various oligosaccharides, and numerous humoral and cellular mediators. These factors seem to increase the development of an infant gut microflora dominated by bifidobacteria and lactobacilli [11].
The frequency of Lactobacillus-positive stool samples was higher than the study of Ahrne, et al. (17-45%) [4]. Ahrne, et al. detected that L. rhamnosus and L. gasseri were the most commonly isolated species from faeces of infants under 2 month age. Also at 6 month of age L. rhamnosus (21%) and L .paracasei (15%), at 12 month of age L. paracasei (7%) and L. fermentum (4%), and at 18 month of age L. paracasei (22%) and L. fermentum were the most commonly isolated species [4].
However, our results were not in accordance with the findings of Ahrne, et al. [4]. In our study the L. casei (36.6%), L. plantarum (23.3%) and L. reuteri (23.3%) were the predominant species in the faeces of infants under 1 month age. In addition L. casei was the most prevalent species isolated from breast-fed infants between 1-18 months, followed by L. plantarum.
Ihekweazu, et al. showed that L. rhamnosus, L. johnsonii and L. paracasei were the predominant species in neonatal gut microflora up to the first month of life [8]. In a study by Arici, et al., L. rhamnosus (33.3%) was the most identified species from the faeces of infants and children under 2 years of age [18]. In a study by Mirlohi, et al. in the Isfahan Province of Iran, the L. acidophilus and L. plantarum were the most recovered species from the faeces of healthy infants between 1 to 19 months [19]. In most above studies, L. rhamnosus was the predominant species in the faeces of healthy infants. Our results, like those of Mirlohi, et al. in Iran, did not show the predominance of L. rhamnosus in the faeces of infants. Variations in Lactobacilli microflora may be due to differences in feeding (breast or formula) and the geographical zone [8,19] or in identification error. Also variations in methodology may account for the differences, since lactobacilli are notoriously difficult to identify by traditional biochemical methods [4].
In conclusion, our results showed the predominance of L. casei and L. plantarum species in stool microflora of breast-fed infants. Some lactobacilli strains have health-promoting effects in the developing infant and used as probiotics. Therefore, further investigation about the probiotic potential of identified species may help in finding of new probiotics.
This work was supported by the Vice-Chancellor for Research grant (no. 21491) of Tehran University of Medical Sciences (Tehran, Iran).
All authors contributed equally.
The authors declare no conflict of interest.
This work was supported by the Vice-Chancellor of Tehran University of Medical Sciences (Tehran, Iran).