The side effect profile of NSAIDs is well-established. For prevention of NSAID-related ulcers, the evidence suggests that misoprostol and PPIs (proton pump inhibitors) are superior to H2RAs (histamine-2-receptor-antagonists). Current guidelines recommend use of the lowest possible NSAID dose, as well as consideration of the patients' gastrointestinal and cardiovascular risk profile.
We assessed the knowledge and prescribing preferences of Internal Medicine (IM) residents with respect to administering gastroprotective agents in patients taking NSAIDs.
A 10-question web-based survey was distributed to several major teaching hospitals in the District of Columbia, Maryland and Virginia area. An online survey software was used to collect and analyze results. Means (with standard deviations) are presented for continuous variables and counts (percentages) are presented for categorical variables. We also performed two univariate logistic regression models to the results. Descriptive statistics for the dataset were stratified according to level of training.
Seven major academic institutions received the survey, with a total of 123 IM trainees responding. Regardless of their level of training, 98 residents (80%) reported that they do routinely assess GI risk factors in those in whom they have prescribed NSAIDs. 85 residents (69%) selected a PPI as the protective agent of choice for at-risk patients. 114 (93%) residents surveyed said they counsel their patients to use acetaminophen products as an alternative to NSAIDs. Senior residents (PGY-3 and PGY-4) answered less likely to assess for GI complications as compared to PGY-1s (OR = 0.87; 95% confidence interval = 1.10 to 5.16, p-value = 0.03).
Despite guidelines regarding the use of gastroprotective agents in patients on NSAID therapy, this practice is still underutilized clinically. Our results demonstrate that the foundation of knowledge is present, yet this is not always carried out in practice. Increased education and continued awareness concerning NSAID-related GI complications should continue throughout training.
Study Highlight Section:
• The side effect profile of NSAIDs is well known. Case-controlled studies and meta-analyses have determined that use of NSAIDs increases the risk for developing peptic ulcers.
• Various strategies exist to decrease the risk of ulcer development including co-prescription of a proton pump inhibitor, H2-receptor antagonist, misoprostol or use of a COX-2 inhibitor.
• To our knowledge, there have been no studies to date that have explored the knowledge base and prescribing practices of Internal Medicine trainees regarding this topic.
• This survey functions as a needs assessment for future interventions to change resident behaviors. It also serves as a needs assessment to further evaluate current opinions and behaviors.
Physician prescribing patterns, NSAIDs, Gastroprotective agents, Proton pump inhibitors (PPI), Misoprostol, Residency training, Medical education, Survey
Non-steroidal anti-inflammatory drugs (NSAIDs), are among the most common drugs causing gastrointestinal adverse events [1,2]. Annually, it has been estimated that 25% of chronic NSAID users for arthritis may develop endoscopic evidence of ulcers [3-5]. Data from case-controlled studies and a meta-analysis concluded that people who use NSAIDs are four times more likely to develop even an uncomplicated peptic ulcer as compared to nonusers of NSAIDs [6-8]. While fewer than 5% of these ulcers bleed or perforate, gastrointestinal bleeding accounts for 200,000-400,000 admissions in the U.S. per year [9]. Over 100,000 admissions are a direct result of bleeding ulcers due to NSAID use [10,11]. It has been estimated that the cost of hospitalization is high, and that 16,500 people die every year from NSAID-related gastrointestinal complications [12]. The socioeconomic impact is therefore substantial. Physicians must weigh the risks and benefits of NSAID therapy carefully.
Practitioners should consider high risk features (a history of a gastrointestinal event, age above 65 years, history of cardiovascular disease, or simultaneous use of an anticoagulant, corticosteroid, aspirin, or a high-dose NSAID) in patients prior to prescribing NSAIDs or gastroprotective agents [3] (Table 1). Furthermore, active Helicobacter pylori infection is an independent risk factor [3]. PPIs, high-dose H2RAs, and full-dose misoprostol (800 mcg/day) are effective options in reducing the risk of ulcer formation [13-29].
Table 1: Risk factors for development of NSAID-related ulcers, per ACG guidelines [3]. View Table 1
Alternatively, the use of COX-2 selective inhibitors, when compared to non-selective NSAIDs, result in fewer ulcers; however, their use is limited by the associated increased risk of poor cardiovascular outcomes [9,10,30-35].
While there have been studies looking at patients' adherence to PPIs while on NSAIDs or prescriber adherence using prescription registry data, we found relatively few studies to date that have assessed provider attitudes or knowledge with regard to prescribing gastroprotective agents with NSAIDs [36-41]. None of these studies were conducted in trainees and they concluded that only a minority of patients are receiving prophylaxis [36-41]. Based on the finding that there is a clear underutilization of this practice, we sought to survey trainees' knowledge and prescribing attitudes on this important topic.
A 10-question web-based survey was designed and distributed via email to internal medicine residents in PGY-1 to PGY-4 at several major teaching hospitals in the District of Columbia, Maryland and Virginia area. These included MedStar Georgetown Hospital, George Washington University Hospital, Walter Reed National Medical Center, Howard University Medical Center, MedStar Union Memorial Hospital, INOVA Fairfax Hospital and Sinai Hospital of Baltimore (Table 2). The survey sought to determine the knowledge base of internal medicine residents regarding their use of gastroprotective agents in patients taking long-term NSAIDs. It also sought to assess their knowledge and opinions as to which NSAIDs were the most and least likely to be associated with GI-related complications and sought to understand what counseling is provided by physicians in training with respect to how patients should take NSAIDs and what alternatives exist.
Table 2: Survey to assess the knowledge of Internal Medicine trainees (by year of training) of the gastrointestinal complications in patients who take chronic NSAIDs. View Table 2
During 2017, the survey was sent on three separate occasions to maximize responses and all results were handled in a confidential manner. An online survey software (Survey Monkey) was used to collect and analyze results. The study was submitted to and approved by the Georgetown Institutional Review Board prior to distribution of the online survey. Means (with standard deviations) are presented for continuous variables and counts (percentages) are presented for categorical variables. We also performed two univariate logistic regression models to the results. Descriptive statistics for the dataset were stratified according to level of training.
The survey was sent to 387 trainees in internal medicine from 7 major teaching hospitals from PGY-1 through 4. A total of 123 responses were collected, (61 PGY-1, 26 PGY-2, 31 PGY-3, 5 PGY-4) for an overall response rate of 32%. PGY-1 (Post-Graduate Year 1) is treated as the reference group for all models presented.
Collectively, 98 residents (80%) reported that they routinely assess for GI risk factors in those in whom they have prescribed NSAIDs. Furthermore, 114 residents (93%) surveyed said they counsel their patients to use acetaminophen products as an alternative to NSAIDs (Table 2).
The majority of residents, 59 (50%) believed that patients are at the highest risk for developing ulcers after 4 or more weeks of daily NSAID consumption. A total of 85 (69%) chose proton pump inhibitors as the gastroprotective agent of choice to prescribe if their patients are deemed to be high-risk. When stratified by level of training, 68% (42 PGY-1), 57% (16 PGY-2), and 78% (28 PGY-3 and PGY-4) of residents in each respective year chose PPI as the medication of choice for gastroprotection (Table 2).
In terms of patient education, when asked how internal medicine residents counsel their patients about taking NSAIDs, the top two responses were: 1) take an NSAID on a full stomach (77.3%), and 2) take a PPI concomitantly as needed (26.8%).
When given a list of the top 10 most commonly prescribed NSAIDs, the residents chose diclofenac, celecoxib, and ketorolac as the three they thought were the least likely to cause ulcers, and ibuprofen, ketorolac, and naproxen as the three most likely. When compared by level of training, they all selected celecoxib as the one they thought was the least harmful. PGY-1 and PGY-2 residents both chose ketorolac as the most harmful, and senior residents (PGY-3 and 4) selected ibuprofen as their choice as the most harmful.
Regarding the top 5 risk factors for developing GI complications, trainees selected the following: a history of ulcers, a history of a bleeding ulcer, high dose NSAID use, use of anti-coagulants or anti-platelets agents, and age over 65. Compared by year of training, PGY-1, PGY-2, and PGY-3 and 4 residents all believed that a history of gastric/duodenal ulcer, a history of a bleeding ulcer, and high dose NSAID use, respectively, were the leading risk factors for NSAID-related GI complications.
For the regression models, we found that senior residents (PGY-3 and 4) were less likely to assess for GI complications as compared to PGY-1s, and this was statistically significant (OR = 0.87; 95% confidence interval = 1.10 to 5.16, p-value = 0.03). In contrast, PGY-2 residents were less likely, and PGY-3 and 4 residents were more likely, as compared to PGY-1 residents to suggest to patients the use of acetaminophen as an alternative to NSAIDs, although this relationship was not significant (p-value = 0.73, and 0.30, respectively).
We attempted to assess the knowledge, clinical perspectives as well as prescribing practices of internal medicine trainees with regard to the use of gastroprotective agents in patients taking NSAIDs chronically. Of the risk factors that the American College of Gastroenterology deem most important in terms of NSAID-related GI complications, all internal medicine residents surveyed, irrespective of their level of training, were able to accurately identify them. They corrected selected a history of an ulcer, a history of a bleeding ulcer, and use of a high dose NSAID as the most important risk factors. Furthermore, all residents irrespective of level of training, were able to accurately identify the time frame when patients are most at risk for ulcer development, which is greater than four weeks after initiation of NSAID therapy. Thus, the results of this survey indicate that the internal medicine trainees surveyed have a solid foundation of knowledge throughout their training. The American College of Gastroenterology (ACG) published guidelines in 2009 to assist physicians in selecting the appropriate treatment while taking into account the gastrointestinal and cardiovascular risks [3]. We created an algorithm for selection of NSAID and co-therapy, adapted from these guidelines (Figure 1).
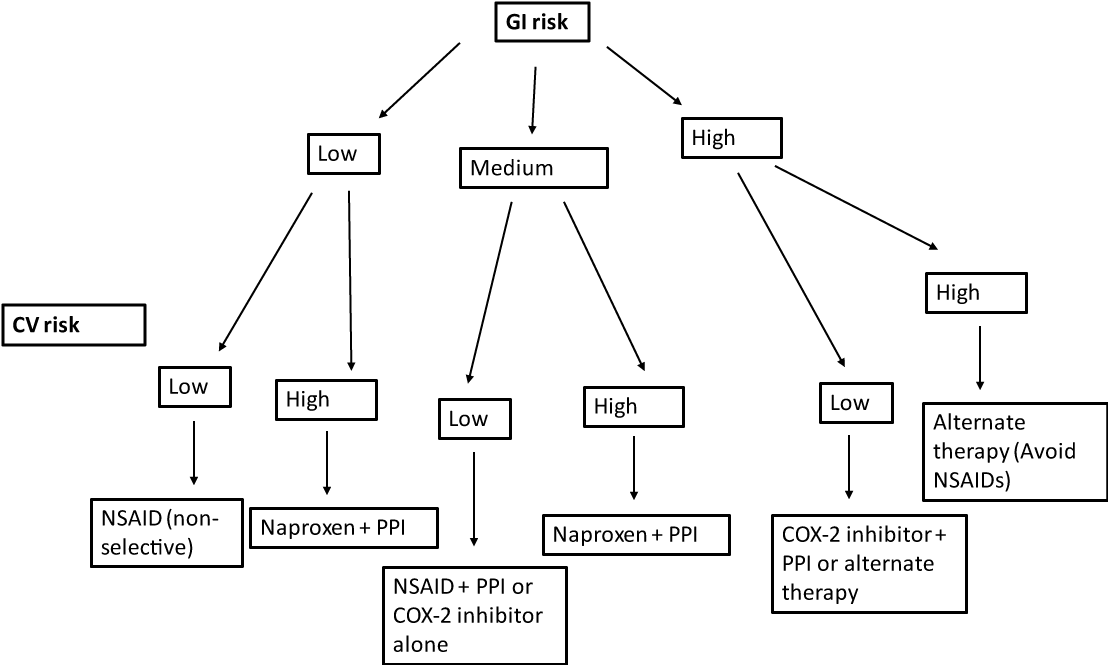 Figure 1: Algorithm for selection of NSAID and co-therapy, adapted from ACG guidelines GI risk: low risk (no risk factors); moderate risk (one or two risk factors); high risk (three of more risk factors, or previous ulcer complications or use of steroids or anticoagulants). Risk factors include: age > 65 years, high dose NSAID therapy, previous complicated ulcer, use of aspirin, steroids, anticoagulants CV risk: high (requiring low dose aspirin). View Figure 1
Figure 1: Algorithm for selection of NSAID and co-therapy, adapted from ACG guidelines GI risk: low risk (no risk factors); moderate risk (one or two risk factors); high risk (three of more risk factors, or previous ulcer complications or use of steroids or anticoagulants). Risk factors include: age > 65 years, high dose NSAID therapy, previous complicated ulcer, use of aspirin, steroids, anticoagulants CV risk: high (requiring low dose aspirin). View Figure 1
Our study showed that there is variability amongst the residents in terms of their knowledge of the gastrointestinal risk of particular NSAIDs and their association with ulcer development. When presented with a list of commonly prescribed NSAIDs, internal medicine residents, when pooled together, chose diclofenac (35.8%), ketorolac (35.8%), and celecoxib (75.6%) as the medications they thought were the least likely to cause GI complications. Ibuprofen (49.6%), ketorolac (52.0%), and naproxen (51.2%) were chosen as the most likely to cause side effects. It is interesting to see that ketorolac appears in the list of the top three most and least harmful medications. The majority of residents selected celecoxib as the safest NSAID, although there was no clear consensus as to which was the most harmful.
The trainees' responses differed from those in a meta-analysis of case-control studies which found that the highest risk NSAIDs were piroxicam and ketoprofen followed by indomethacin, naproxen, diflunisal, sulindac, diclofenac, and ibuprofen, ranked from highest to lowest risk [42] (Table 3). Another review of observational studies found that when using upper GI bleed or perforation as the endpoint, the highest to lowest risk NSAIDs were ketorolac, piroxicam, naproxen, ketoprofen, indomethacin, meloxicam, diclofenac and ibuprofen [43] (Table 3). The results from this survey are mixed in terms of concordance with prior studies, as our residents chose ketorolac and naproxen as having the most harmful GI side effects, and diclofenac and celecoxib as being the most benign. It should be noted that the observational study and meta-analysis cited above did not include COX-2 inhibitors in their analysis. Coté, et al. found that structured, formal patient education in conjunction with computer alerts to remind patients to take their medications, could improve overall long-term gastroprotection in at risk NSAID users [44].
Table 3: Ranking of gastrointestinal-related toxicity of commonly prescribed NSAIDs. View Table 3
The most commonly chosen response when asked about patient counseling in our survey was to advise patients to take NSAIDs on a full stomach. However, the responses with regard to when to take the NSAID or if a PPI should be taken concomitantly were more variable. When asked about what agent they would prescribe, the majority of internal medicine residents in every group chose a PPI over misoprostol or an H2RA. Although our study is the first, to our knowledge, to assess trainee perspectives, a study in Japan of gastroenterologists and orthopedists, found that for their high-risk patients, the majority of these clinicians also chose to prescribe a PPI over an H2RA [38]. Misoprostol can decrease the risk of GI complications by 40% but is limited by its own adverse effect profile of diarrhea, cramping, and the risk as an abortifacient [45]. PPIs are very well-studied and have been associated with decreased risk of bleeding ulcers in patients taking celecoxib, in those with H. pylori, and for NSAID-related ulcers based on epidemiological data [36].
In another Japanese study, investigators created three simulation cases and surveyed 79 gastroenterologists and 234 orthopedists. For high-risk patients, only 67% of gastroenterologists and 43% of orthopedists indicated that they would prescribe a PPI along with an NSAID; and 25% of both groups indicated that they would prescribe a Histamine-2-receptor antagonist [37]. A third Japanese survey of 208 orthopedists found that only 10.8% of these practitioners chose to co-prescribe a gastroprotective agent, but their agent of choice was a PPI [38]. In 2003, a survey by Chey, et al. of 1000 US primary care physicians found that for patients at high risk for GI toxicity, they would recommend a PPI and COX-2 inhibitor 50% of the time [39]. The same investigators re-surveyed these primary care physicians in 2008. After this 5-year interval, 31% of physicians reported prescribing an NSAID more frequently but this group was 52% more likely to give a gastroprotective agent with an NSAID than in 2003. However, they were less likely to give a COX-2 inhibitor in 2008 than in 2003, largely based on the fact that 41% believed that Rofecoxib would increase cardiovascular risk [40]. Finally, a survey in 2004 of general practitioners in France concluded that 4.8% of patients prescribed rofecoxib, versus 2.1% of patients prescribed a non-selective NSAID, had a history of ulcer disease or gastrointestinal bleeding, thus practitioners were more likely to prescribe rofecoxib to those at high risk for NSAID complications [41].
The FDA has approved the use of PPIs for prevention of NSAID-related ulcers along with other indications [46]. Recently, some observational studies have implicated long term use of PPIs to a variety of comorbidities, including dementia, bone fractures, heart disease, clostridium difficile infection, pneumonia, anemia, and small intestinal bacterial overgrowth [46,47-55]. A review of the adverse effect data of PPIs by Vaezi and colleagues concluded that only moderate strength evidence existed for the association between PPI use and bacterial infections [46]. Other risks were based on weak evidence and confounded by problems related to study design [46]. Nevertheless, it is recognized that many patients are taking PPIs for what may be an inappropriate indication [51]. Patients who qualify for long term PPI use, including the prevention of NSAID-related GI complications and erosive esophagitis should always have a discussion about benefits and harms [51,55].
Standard dosing of H2RAs can reduce the risk of duodenal ulcers, but higher dosages are needed to decrease the risk of both gastric and duodenal ulcers [13]. A seminal case-controlled study by Targownik, et al., compared all possible combinations of protection (NSAID and PPI, NSAID and misoprostol, COX-2 selective inhibitor, COX-2 selective inhibitor and PPI) and found that all four groups were associated with statistically significant reductions in the risk of upper GI complications including ulcer formation, with the greatest risk reduction being from the co-administration of a PPI along with a COX-2 selective inhibitor [19]. From this study, we can extrapolate that there are multiple approaches to selection of the safest regimen. As no two patients are alike, a tailored regimen should be created based on the individual's risk profile. However, this study highlights the fact that a PPI co-administered with a COX-2 selective inhibitor may actually provide the highest benefit in terms of risk reduction for development of ulcers.
Not all clinical trials have shown a clear-cut benefit to the use of PPIs co-prescribed with an NASID. For example, the CLASS study, compared patients with arthritis who took celecoxib at two-to-four times the recommended maximal daily dose to those taking ibuprofen or diclofenac (both of which are non-selective NSAIDs) [9]. They found that the latter two were associated with non-significant higher rates of ulcer and ulcer complication development including gastrointestinal bleeding, perforation, or gastric outlet obstruction [9] (Table 4). However, the annual incidence of UGI ulcer complications plus symptomatic ulcers was higher in the non-selective NSAID group compared to celecoxib, and this was statistically significant [9]. Aspirin when added to celecoxib resulted in a higher relative risk of ulcer complications as compared to celecoxib alone [9].
Table 4: Summary of relevant trials studying the GI complications of NSAID therapy. View Table 4
Similarly, the VIGOR study compared rofecoxib, a COX-2 inhibitor, against naproxen in patients with rheumatoid arthritis [10]. While excluding patients taking aspirin similar to the CLASS study, these investigators found that those taking rofecoxib had a 60% reduction in GI perforations, obstruction and bleeding [10]. In contrast, a study by Ashcroft, et al., patients with arthritis whom were treated with celecoxib, were surprisingly found to have gastric or duodenal ulcers, at a wide range of doses, from 100 to 800 mg per day [56]. This was a systematic review of five randomized controlled trials of endoscopically identified gastric and duodenal ulcers in patients with osteoarthritis or rheumatoid arthritis in people taking celecoxib versus diclofenac, ibuprofen, naproxen, or placebo [57]. At 12 weeks, compared to placebo, the pooled rate ratio of endoscopic ulcers for celecoxib at 100 mg twice a day was 1.96 (not statistically significant), and at 200 mg twice a day was 2.35 (statistically significant).
Based on their responses to this survey, trainees have an incomplete understanding of the risk of individual NSAIDs causing ulcers compared to studies in the literature. However, they are able to accurately identify the factors that place patients at risk for developing ulcers. Senior trainees were also more likely to counsel patients on alternatives to NSAIDs as compared to PGY-1s, although this was not statistically significant. Further education needs to begin in medical school and continue throughout residency training. Future studies should survey subspecialists who prescribe long-term NSAIDs.
Anagha Kumar, MA, MS for statistical analysis.
None.
No financial disclosures.