To evaluate the use of antivirals in pregnancy to prevent vertical transmission of chronic hepatitis B in a gastroenterology practice specializing in treatment of pregnant women.
Retrospectively identified 226 pregnant women with chronic hepatitis B seen by hepatologists at the Center for Women's Gastrointestinal Health at Women and Infants Hospital, Providence, Rhode Island from January 1, 2009 - December 31, 2014. 150 met inclusion criteria.
13% (19) women had HBV DNA levels > 1,000,000 IU/ml in the third trimester of pregnancy which qualified them for initiation of treatment for chronic hepatitis B to prevent vertical transmission. However, 10 of the eligible 19 patients (53%) were not initiated on treatment during pregnancy to prevent vertical transmission. 7 of the 10 patients (64%) were never offered antiviral therapy by their hepatologist during their third trimester to prevent vertical transmission. 3 of the 10 patients declined (27%) antiviral prophylaxis. 9 of the 19 women (47%) initiated antiviral therapy in third trimester to prevent vertical transmission: Five were initiated on tenofovir, three on lamivudine, and one on telbivudine. HBIG and vaccines were successfully administered in a timely fashion in all 93 (100%) infants for whom this data was available.
This study reveals a lack of consistency in physician antiviral use for highly viremic mothers in their third trimester. Our finding highlights the urgent need for clearly defined consensus guidelines from the liver societies on the threshold HBV DNA level for initiation of antivirals in pregnancy.
Hepatitis B, Pregnancy, Antiviral use, Vertical transmission, Adherence, Treatment guidelines
Chronic hepatitis B affects approximately 1.25 million persons in the United States, leading to significant morbidity and mortality from liver cirrhosis and hepatocellular carcinoma [1]. The risk of perinatal transmission of hepatitis B to infants born to HBsAg-positive mothers has been reported to be as high as 90 percent without the use of active and passive immunization [2]. Since the introduction of universal maternal screening programs, vaccination of all newborns, and the use of prophylactic hepatitis B immune globulin (HBIG) for infants of HBsAg-positive mothers, we have reduced vertical transmission rates from 90 percent to as low as 5 to 10 percent [3,4]. Vertical transmission risk is significantly associated with high HBV DNA level > 1,000,000 IU/mL in the mothers, who are often e-antigen positive (replicative phase). In these highly viremic women, perinatal transmission rates are still as high as 10% even with neonatal immunization and immuno prophylaxis [5-8].
In mothers who are not already on treatment, antiviral use (in addition to standard passive-active immunization of the neonate) in women with high HBV DNA levels (> 1,000,000 IU/mL) starting at 28-32 weeks gestation further reduces the risk of perinatal transmission. Numerous studies have shown that use of antiviral agents in pregnant women are safe, and further decreases vertical transmission rates by reducing maternal viral load [8-11]. Tenofovir is the preferred antiviral therapy in pregnancy because resistance is rare. A recent prospective study of the efficacy of tenofovir in reducing perinatal transmission showed that newborns born to mothers who received Tenofovir had significantly lower rates of HBsAg positivity at six months (1.5 versus 10.7 percent) [12]. Other agents (e.g, lamivudine, telbivudine) are also safe in pregnancy for reducing perinatal transmission [8-11]; however, they are associated with increased rates of antiviral resistance.
This study aimed to evaluate our use of antivirals in the third trimester in a large obstetric center with a specialized gastroenterology practice that has expertise in treating pregnant women. We also examined barriers to adherence to other obstetric recommendations in preventing vertical transmission of hepatitis B such as timely administration of active and passive immunoprophylaxis to newborns within 12 hours of birth.
This is the first study to examine rates of and barriers to antiviral use in highly viremic mothers to prevent vertical transmission of hepatitis B.
We retrospectively identified 226 pregnant women (age > 18) with chronic hepatitis B (defined as positive hepatitis B surface antigen or detectable hepatitis B virus DNA for at least 6 months) seen by hepatologists at the Center for Women's Gastrointestinal Health at Women and Infants Hospital, Providence, Rhode Island from January 1, 2009 - December 31, 2014. Medical records were reviewed for demographic and clinical information from initial presentation for positive hepatitis B surface antigen during pregnancy until 6 months postpartum. 150 met inclusion criteria. 57 were excluded for incomplete data in medical records, 10 were excluded for coinfection with hepatitis C, or HIV, and 3 were excluded for clearing hepatitis B before the study period. 6 patients who were already on treatment for hepatitis B prior to pregnancy were excluded.
Each patient chart including labs, imaging, and clinic notes were analyzed in detail by two independent reviewers to ensure accuracy. In the event of discordant conclusions, a third reviewer (a senior hepatologist) adjudicated. Pertinent information was recorded into a patient database.
The primary outcome was whether an antiviral was initiated in pregnant women with HBV DNA greater than 1,000,000 IU/ml at 28-32 weeks gestation. The secondary outcome was reasons for non-initiation of antivirals in those who met this DNA threshold.
This study was approved by the Institutional Review Board of Women and Infants hospital.
Patient demographic and clinical characteristics for the 156 study patients are shown in Table 1. Median age was 28 years (IQR 21, 35). Only 5% (7) patients were born in the US, and 91% (137) were foreign born, and 4% (6) did not state country of birth. 4% (6) women had ALT levels more than twice the upper limit of normal for women (19 U/L) at any point during their pregnancy. 13% (20) women were hepatitis B e-antigen positive.
Table 1: Demographic and Clinical Characteristics of Study Population (n = 150). View Table 1
13% (19) women had HBV DNA levels > 1,000,000 IU/ml in the third trimester of pregnancy, which qualified them for initiation of treatment for chronic hepatitis B to prevent vertical transmission.
However, as Figure 1 and Table 2 show, 10 of the eligible 19 patients (53%) were not initiated on treatment during pregnancy to prevent vertical transmission.
 Figure 1: Antiviral Use Third Trimester (n = 19).
View Figure 1
Figure 1: Antiviral Use Third Trimester (n = 19).
View Figure 1
Table 2: Antiviral Use Third Trimester (n = 19). View Table 2
7 of the 10 patients (64%) were never offered antiviral therapy by their hepatologist during their third trimester to prevent vertical transmission (Table 3). 3 of the 10 patients declined (27%) antiviral prophylaxis (Figure 2 and Table 3).
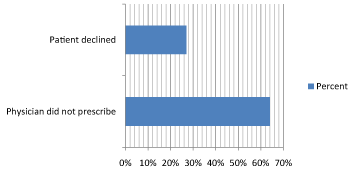 Figure 2: Reasons for Non-Initiation of Antiviral Prophylaxis in Third Trimester (n = 10).
View Figure 2
Figure 2: Reasons for Non-Initiation of Antiviral Prophylaxis in Third Trimester (n = 10).
View Figure 2
Table 3: Reasons for Non-Initiation of Antiviral Prophylaxis in Third Trimester (n = 10). View Table 3
9 of the 19 women (47%) initiated antiviral therapy in third trimester to prevent vertical transmission: Five were initiated on tenofovir, three on lamivudine, and one on telbivudine.
HBIG and vaccines were successfully administered in a timely fashion in all 93 (100%) infants for whom this data was available (Table 4).
Table 4: Obstetric characteristics. View Table 4
This is the first study to examine adherence to antiviral use in highly viremic mothers to prevent vertical transmission of hepatitis B. Our study reveals that lack of physician initiation of antivirals was the primary reason that pregnant women at the highest risk of vertical transmission of hepatitis B did not receive antiviral prophylaxis, rather than patient refusal due to fear of medication risks to their fetus, or patient loss to follow-up.
The majority of pregnant women who had very high viral loads in their third trimester, and thus who would have benefitted most from antiviral use, were never offered antiviral therapy by their hepatologist. There was a lack of consistency across the practice among the different physicians in initiation of antivirals for pregnant women with HBV DNA > 1,000,000 IU/ml. One possible reason for this discrepancy is a lack of official guidelines from the American Association for the Study of Liver Disease (AASLD) for management of hepatitis B in pregnancy [13] until very recently in 2015. Up until very recently, the AASLD guidelines (2009) for chronic hepatitis B made no mention of management of hepatitis B in pregnancy [13]. In contrast, the European Association for Study of the Liver (EASL) hepatitis B guidelines have recommended use of antivirals to decrease risk of vertical transmission in highly viremic mothers (serum HBV DNA > 10^6 or 10^7 IU/ml) [14]. The Asian Pacific Association for the Study of the Liver (APASL) hepatitis B guidelines acknowledge that numerous studies have shown that antiviral therapy administered in third trimester further reduces the risk of vertical transmission from highly viremic mothers, as compared with passive-active immunization alone, so there is no controversy on the benefit of antiviral effectiveness in reducing vertical transmission.
However, there has not been consensus across the three largest liver disease societies in the world (AASLD, EASL, APASL) on the threshold of serum HBV DNA level for initiating therapy. This threshold remains imprecisely defined (ranging from 10^6 IU/ml to 10^8 IU/ml), which unfortunately can lead to inconsistent initiation of antivirals as this study clearly shows. Furthermore, one recent study examining actual treatment rates of treatment eligible chronic hepatitis B patients in the community showed that "physicians are inclined to take a more conservative approach in determining patients' treatment eligibility" [14]. Without a clear threshold HBV DNA level for antiviral initiation, physicians may be reluctant to initiate antiviral therapy in pregnancy.
This study has limitations inherent to its retrospective design. For example, the study design did not allow a better understanding of why a few patients declined therapy despite physician recommendation of treatment for very high viral loads. The single center nature of the study may limit its generalizability to other gastroenterology settings. Furthermore, the small sample size precluded multivariate analyses.
Although we have achieved excellent rates of screening for hepatitis B in prenatal care in the US, and there is no controversy on the benefit of antiviral effectiveness in reducing vertical transmission in highly viremic mothers, this study found that a large number of these women are not being offered antiviral therapy by their physicians. This study reveals a lack of consistency in physician antiviral use for highly viremic mothers in their third trimester. Our finding highlights the urgent need for clearly defined consensus guidelines from the liver societies on the threshold HBV DNA level for initiation of antivirals in pregnancy.