Investigation of gastric and colon microbiota in Helicobater pylori-infectes patients shown that: 1. Gastric microbiota in H. pylori-positive and H. pylori-negative patients was different and the samples from H. pylori-positive patients were characterized by the higher growth of opportunistic bacteria; 2. The link between H. pylori in stomach and colon microbiota is possible; 3. After eradication without probiotics we saw decreasing level of Bifidobacteria spp., Lactobacillus spp. and increasing level of opportunistic bacteria and Candida albicans but in case of additional usage of probiotics the colon microbiota is better after treatment. Perhaps it is needing to perform future studies to confirm our results.
Helicobacter pylori, Microbiota, Probiotics
It is well known that Helicobacter pylori infection is not the only microorganism colonizing the gastric mucosa and other microorganisms can affect stomach mucosa [1-5]. Disorders of gastric and colon microbiota (and its role in gastroenterology diseases) in patients infected with Helicobacter pylori are widely investigated [5-9]. Gastric colonization with H. pylori induces histologic gastritis in all infected individuals, but the disease remains asymptomatic as only a minority of patients develop any apparent clinical signs of this colonization H. pylori-positive patients have a risk of developing ulcer disease and gastric cancer [10 ]. Eradication therapy with antibiotics leads to worsening of microbiota of digestive tract [11,12], that's why it is important to find ways for prophylaxis of dysbiotic changes in microflora after anti-helicobacter treatment, for example, probiotics usage [9,13-17]. The potential of probiotic lactic acid bacteria as additional therapy of H. pylori infection is no doubt. These beneficial bacteria can correct gastrointestinal dysbiosis during H. pylori eradication [5,6].
1. To compare gastric microbiota of H. pylori positive and H. pylori negative patients.
2. To find possible correlation between colon microbiota (content of Bifidobacteria, Lactobacilli, Enterococci and Candida albicans in stool) and amount of H. pylori in stomach mucosa.
3. To compare colon microbiota before and after eradication with additional usage of probiotic.
Gastric microbiota: Clinical study was performed on the group of 11 patients (5 men and 6 women, 53.8 ± 20.7) with dyspepsia. During the gastro-duodenoscopy, a biopsy was taken from the gastric body, gastric antrum, and duodenum of all the patients. H. pylori infection was verified by polymerase chain reaction (PCR) and bacteriological method. Gastric microbiota was analyzed employing PCR in real time (PCR-RT). The members of family Herpesviridae (Herpes simplex virus, Cytomegalovirus, Epstein-Barr virus) detection was performed by AmpliSens® Multiplex Real-Time Kits. It was a pilot study with a small number of patients to analysis the gastric microbiota by Real-Time PCR.
Colon microbiota: 103 persons infected with H. pylori were observed (30 men and 73 women, 45.9 ± 11.6). For all patient's gastroscopy with biopsies from a stomach body and antrum were performed for verification of H. pylori infection (rapid urease test, PCR and histological method). Amount of H. pylori was estimated in stomach body and antrum by evaluation quantity of microbes (microscopic analysis): < 20 microbes - mild (1 grade), 20-50 - moderate (2 grade), > 50 - high (3 grade). Bacteriological analysis of stool was performed to evaluate the content of microorganisms in the colon (lgCFU/g). Designing our study, we considered the best possible combination of tests and methodologies to achieve best results. Overall, given our study design we found possible to achieve proper precision with bacteriological analysis, but we are looking forward to performing additional research applying metagenomics methodologies.
Microbiota after eradication: 75 patients (24 men and 51 women, 38.3 ± 9.4) took standard triple eradication therapy for 10 days (omeprazole 20 mg, clarithromycin 500 mg, amoxicillin 1000 mg twice a day). 1st group of patients (n = 23) in additionally received probiotic (Enterococcus faecium strain L-3), 2nd group (n = 32) - lyophilisate of the cultural fluid of Bacillus subtilis, 3rd group (n = 20) - only standard therapy. Verification of H. pylori infection was made with rapid urease test, PCR and histological method, bacteriological analysis of stool was performed to evaluate the content of microorganisms in colon (lgCFU/g) before and 1-1.5 month after treatment. In different groups number of patients was not the same because after the course of therapy some patients lost from follow up and didn't come to visit for eradication control. We estimated the results only for those patients who came to visits before and after treatment. We choose the two types of probiotic strains (Enterococcus faecium strain L-3, Bacillus subtilis) because these strains are commonly used in Russia and in previous in vitro studies we saw a direct inhibitory effect of these strains against H. pylori.
Statistical analysis was performed using MS Excel (Microsoft, Redmond, WA, USA) and Statistica 6.0 (StatSoft, Tulsa, OK, USA) for Windows XP.
Gastric microbiota: 5 patients under study were infected with H. pylori (45.5%), 6 patients were H. pylori negative (54.5%). H. pylori were isolated from stomach (27.27%) and stomach and duodenum simultaneously (18.18%). The members of family Herpesviridae were not detected (Figure 1).
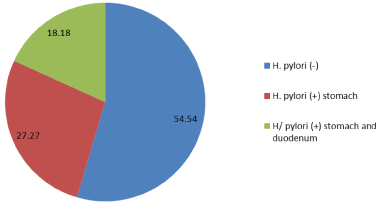 Figure 1: Frequency of isolation H. pylori in the stomach and duodenum. View Figure 1
Figure 1: Frequency of isolation H. pylori in the stomach and duodenum. View Figure 1
Gastric microbiota in these two groups of patients were different. Bacteria of the genera: Proteus, Klebsiella, Enterobacter or atypical E. coli were determined only in the samples from H. pylori-positive patients (Figure 2 and Figure 3).
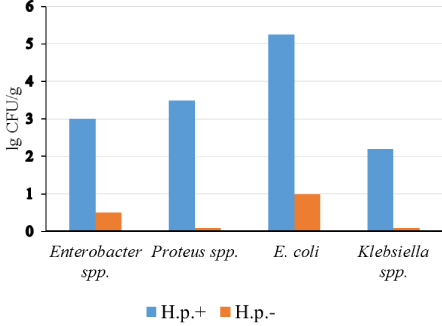 Figure 2: The content of most important enterobacteria in gastric microbiota H. pylori (+) and H. pylori (-) patients (p < 0.05).
Figure 2: The content of most important enterobacteria in gastric microbiota H. pylori (+) and H. pylori (-) patients (p < 0.05).
Axis X: Type of bacteria; Axis Y: The content of the bacteria, lgCFU/g.
View Figure 2
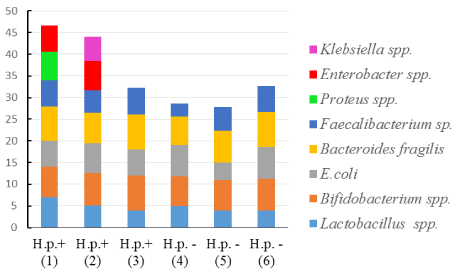 Figure 3: The ratio (%) of the most important bacteria in the gastric microbiota composition H. pylori (+) and H. pylori (-) patients.
Figure 3: The ratio (%) of the most important bacteria in the gastric microbiota composition H. pylori (+) and H. pylori (-) patients.
Axis X: Type of bacteria; Axis Y: The ratio of the bacteria, %.
View Figure 3
Colon microbiota: Baseline characteristics of colon microbiota for all patients presented in Table 1. Amount of H. pylori in stomach body was associated with the lower content of Bifidobacteria spp. and higher content of Candida albicans in colon (Figure 4 and Figure 5).
Table 1: Baseline characteristics of colon microbiota in H. pylori-infected patients. View Table 1
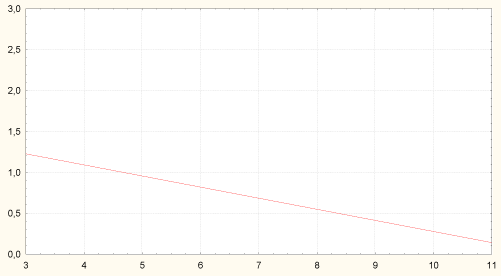 Figure 4: Correlation between amount of H. pylori in stomach body and content of bifidobacteria in colon (r = -0.29, p = 0.029). Axis of abscises - content of Bifidobacteria spp. in colon, lgCFU/g; axis of ordinates - amount of H. pylori in stomach body, grade 1-3.
Figure 4: Correlation between amount of H. pylori in stomach body and content of bifidobacteria in colon (r = -0.29, p = 0.029). Axis of abscises - content of Bifidobacteria spp. in colon, lgCFU/g; axis of ordinates - amount of H. pylori in stomach body, grade 1-3.
Axis X - content of Bifidobacteria spp. in colon, lgCFU/g; Axis Y - amount of H. pylori in stomach body, grade 1-3.
View Figure 4
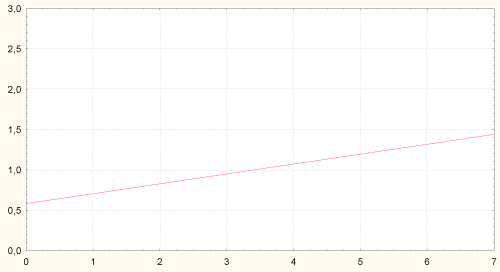 Figure 5: Correlation between amount of H. pylori in stomach body and content of Bifidobacteria in colon (r = -0.29, p = 0.029). Axis of abscises - content of Bifidobacteria spp. in colon, lgCFU/g; axis of ordinates - amount of H. pylori in stomach body, grade 1-3.
Figure 5: Correlation between amount of H. pylori in stomach body and content of Bifidobacteria in colon (r = -0.29, p = 0.029). Axis of abscises - content of Bifidobacteria spp. in colon, lgCFU/g; axis of ordinates - amount of H. pylori in stomach body, grade 1-3.
Axis X - content of Bifidobacteria spp. in colon, lgCFU/g; Axis Y - amount of H. pylori in stomach body, grade 1-3.
View Figure 5
Amount of H. pylori in stomach antrum was associated with higher content of Candida albicans in colon and degree of colon dysbiosis (Figure 6).
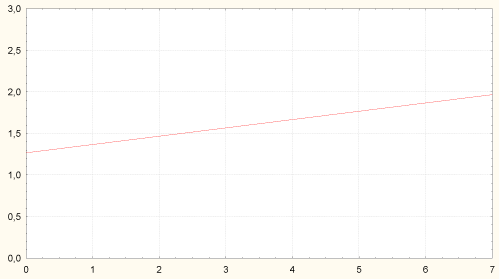 Figure 6: Correlation between amount of H. pylori in stomach antrum and content of Candida albicans in colon (r = 0.30, p = 0.006). Axis of abscises - content of Candida albicans in colon, lgCFU/g; axis of ordinates - amount of H. pylori in stomach body, grade 1-3.
Figure 6: Correlation between amount of H. pylori in stomach antrum and content of Candida albicans in colon (r = 0.30, p = 0.006). Axis of abscises - content of Candida albicans in colon, lgCFU/g; axis of ordinates - amount of H. pylori in stomach body, grade 1-3.
Axis X - content of Candida albicans in colon, lgCFU/g; Axis Y - amount of H. pylori in stomach body, grade 1-3.
View Figure 6
Microbiota after eradication: Eradication rate was significantly higher in 1st and 2nd groups (Figure 7). After eradication, we saw a decrease of Bifidobacteria spp., Lactobacillus spp. and an increase of semi-pathogens microorganisms and Candida albicans in 3rd group (Table 2). It can be associated with intake of antibiotics without probiotics.
Table 2: Colon microbiota before and after eradication (lgCFU/g. M ± m). View Table 2
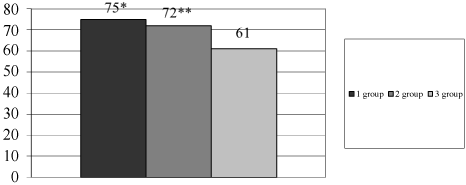 Figure 7: Eradication rate in different groups of patients.
Figure 7: Eradication rate in different groups of patients.
*- р < 0.05 - difference is significant between 1st and 3rd group; **- р < 0.05 - difference is significant between 2nd and 3rd group; Axis X - groups of patients; Axis Y - eradication rate, %.
View Figure 7
1. Gastric microbiota in H. pylori-positive and H. pylori-negative patients appeared to be different. The samples from H. pylori positive patients were characterized by the higher growth of opportunistic bacteria. Future scientific analysis is needed to investigate the role of gastric microorganisms in pathology of digestive system.
2. According to our study the link between H. pylori and colon microbiota is possible. A further research is needed to confirm this early finding.
3. After the eradication we saw laboratory signs of colon dysbiosis: Decreasing level of Bifidobacteria spp., Lactobacillus spp. and increasing level of opportunistic bacteria and Candida albicans. All symptoms appear to worsen for those patients without probiotic therapy as a parallel to eradication course. In case of a supplementing eradication therapy with probiotics, the eradication rate was higher and colon microbiota was more stable after treatment than in patients who took PPI and antibiotic only. An application of probiotics, in this case, can have several effects: Protection effect on colon microbiota and prevention of dysbiosis, synthesis of bacteriocins with direct inhibitory effect against H. pylori, positive impact on the human immune system due to correction of colon microbiota. Summing-up described application of probiotics can be a promising method to improve eradication efficacy and safety in anti-helicobacter therapy.
4. We found a possibility of colon microbiota being possibly associated with different diseases besides of a digestive system. Consequently, it is important to search different methods to correct, improve and maintain the balance of colon microbiota. One of these methods can be probiotics application.
This work was supported by the Russian Science Foundation №16-15-10085.