Pancreatitis is a common adverse reaction to asparaginase and a reason for drug discontinuation in patients with acute lymphoblastic leukemia. Diagnostic criteria include elevated pancreatic enzymes, abdominal symptoms and/or imaging findings suggestive of pancreatitis. There is no specific treatment, but in a few reports, octreotide seems to be an effective drug in reducing acute complications and mortality. Here we describe the case of a child who developed asparaginase-induced necrotizing pancreatitis, successfully treated with octreotide. A review of the literature indicated that evidence of necrotizing pancreatitis markedly increases the risk of acute and chronic complications, and patients who received octreotide usually improved without surgical intervention.
Necrotizing pancreatitis, L-Asparaginase, Octreotide, Acute lymphoblastic leukemia
ALL: Acute Lymphoblastic Leukemia; AAP: Asparaginase-Associated Pancreatitis; EGIL: European Group of Immunological Markers for Leukemia; CT: Computerized Tomography; PTWG: Ponte di Legno Working Group; CTCAE: Common Terminology Criteria for Adverse Events
Asparaginase is an essential drug in the treatment of acute lymphoblastic leukemia (ALL); it causes the depletion of serum asparagine concentrations by way of hydrolysis into aspartic acid and ammonia, resulting in reduced protein synthesis and, finally, the apoptosis of leukemic cells [1].
Adverse reactions to asparaginase include clinical and subclinical hypersensitivity, hepatic dysfunction, hypertriglyceridemia, hyperglycemia, thrombosis, myelosuppression, encephalopathy, and pancreatitis [1]. Asparaginase-associated pancreatitis (AAP) is a fairly common event. Studies assessing AAP retrospectively show a variable incidence rate (between 6-7% and 18%) [2,3], depending on the different definitions of AAP [4]. Necrotizing pancreatitis is a severe form of pancreatitis, almost always associated with local and systemic complications and with a high risk of multiorgan failure, requiring intensive medical therapy [5]. Octreotide, a somatostatin analog, has been used for the treatment of AAP and severe acute pancreatitis from other causes in children [6]: it seems to reduce acute complications and mortality [7].
Here we describe the case of a child with ALL who developed necrotizing pancreatitis following asparaginase administration, successfully treated with octreotide; thereafter, a literature review on this topic is reported.
A previously healthy 3-year-old boy came to our attention because of fever for three days, cervical lymphadenopathy, and hepatosplenomegaly. A complete blood count showed hemoglobin 6.9 g/dl, white blood count 336,050/µl, and platelets count 56,300/µl. A subsequent bone marrow aspirate along with cytofluorimetric analysis revealed almost complete replacement of normal hematopoietic elements by lymphoid blasts, positive for CD10, CD19, CD45, consistent with a diagnosis of a B-lineage ALL (European Group of Immunological Markers for Leukemia B-II subtype). No leukemic cells were found in the cerebrospinal fluid. The patient was enrolled in the AIEOP-BFM ALL 2017 protocol and began the induction phase, which consists of 28 days of prednisone (60 mg/m 2 /day), 4 weekly doses of vincristine (1.5 mg/m 2 /dose) and daunorubicin (30 mg/m 2 /dose) on day 8, 15, 22 and 29, and two doses of PEG-asparaginase (2,500 IU/m 2 /dose) on day 12 and 26.
On the 22 nd day of induction treatment, ten days after the first dose of PEG-asparaginase, the boy developed severe abdominal pain and was immediately admitted to our Unit. On physical examination, abdominal tenderness was detected, without any peritoneal signs; the child was also febrile, while other vital signs were normal. Due to a sudden decrease in urine output, a bladder catheter was promptly inserted. Blood tests showed an increased level of amylase (1,316 U/l, normal range 28-100 U/l) and lipase (2,149 U/l, normal range 13-60 U/l). An abdominal ultrasound was performed: the pancreas was not visualized because of overlying bowel gas, but intense edema of peripancreatic fat was recognized, with abundant peritoneal effusion. A diagnosis of AAP was made and prompt management with fasting, parenteral nutrition, albumin replacement, pain control (intravenous tramadol), and antibiotic therapy (cefepime) was started; the prednisone dose was also tapered. Despite this, the boy deteriorated clinically: he became tachypneic, with low oxygen saturation (90%) and multiple laboratory abnormalities, indicative of liver and kidney failure. On the third day, he developed ecchymosis around his umbilicus and on his left flank (Figure 1). A chest-abdomen computerized tomography (CT) was performed: an enlargement of the pancreas with multiple areas of lack of contrast enhancement within the body and the tail was seen, compatible with a diagnosis of necrotizing pancreatitis; furthermore, peripancreatic fluid collections, a large amount of intra-abdominal effusion in supra- and sub-mesocolic spaces, and soft-tissue edema of the anterior abdominal wall with fluid collection in the periumbilical area and in the right abdominal wall were detected, in association with right pleural effusion (Figure 2). He was transferred to the Pediatric Intensive Care Unit; octreotide infusion was promptly initiated at 1 µg/kg/hour; based on positive fluid balance, the boy received furosemide, and because of hyperglycemia, insulin infusion was started. The clinical conditions gradually improved: after seven days, amylase and lipase decreased (280 U/l and 201 U/l, respectively), blood glucose level normalized and insulin was discontinued; a new chest-abdomen CT revealed the persistence of pancreatic edema, with a size reduction of the hypoperfusion areas, and the appearance of a 3 × 2 cm hypodense lesion in the pancreatic body, indicative of pseudocyst (Figure 3).
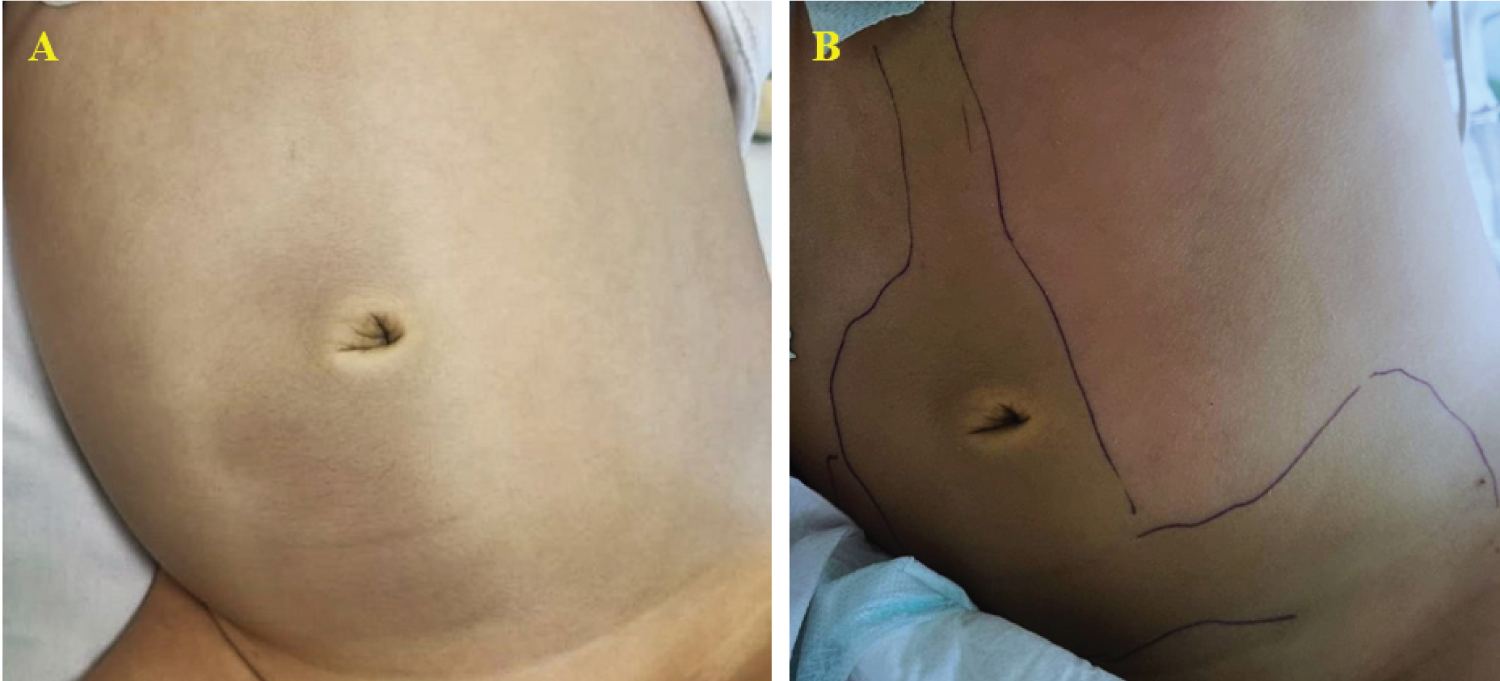 Figure 1: (A,B) Cullen and Grey-Turner signs: periumbilical and flank ecchymosis, suggestive of necrotizing pancreatitis.
View Figure 1
Figure 1: (A,B) Cullen and Grey-Turner signs: periumbilical and flank ecchymosis, suggestive of necrotizing pancreatitis.
View Figure 1
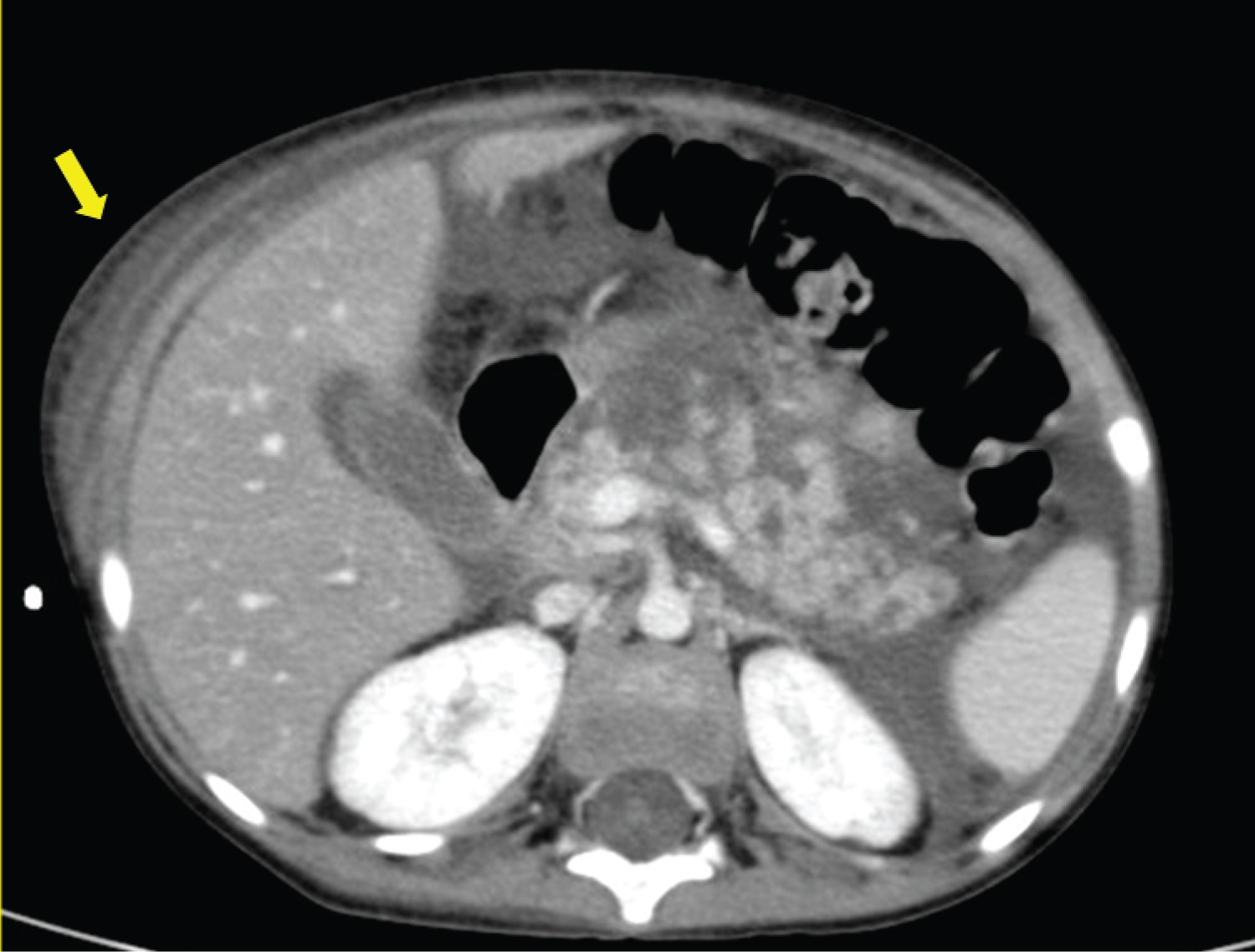 Figure 2: Abdomen CT: enlargement of the pancreas with multiple areas of hypoperfusion after contrast injection within the body and the tail, compatible with a diagnosis of necrotizing pancreatitis.
View Figure 2
Figure 2: Abdomen CT: enlargement of the pancreas with multiple areas of hypoperfusion after contrast injection within the body and the tail, compatible with a diagnosis of necrotizing pancreatitis.
View Figure 2
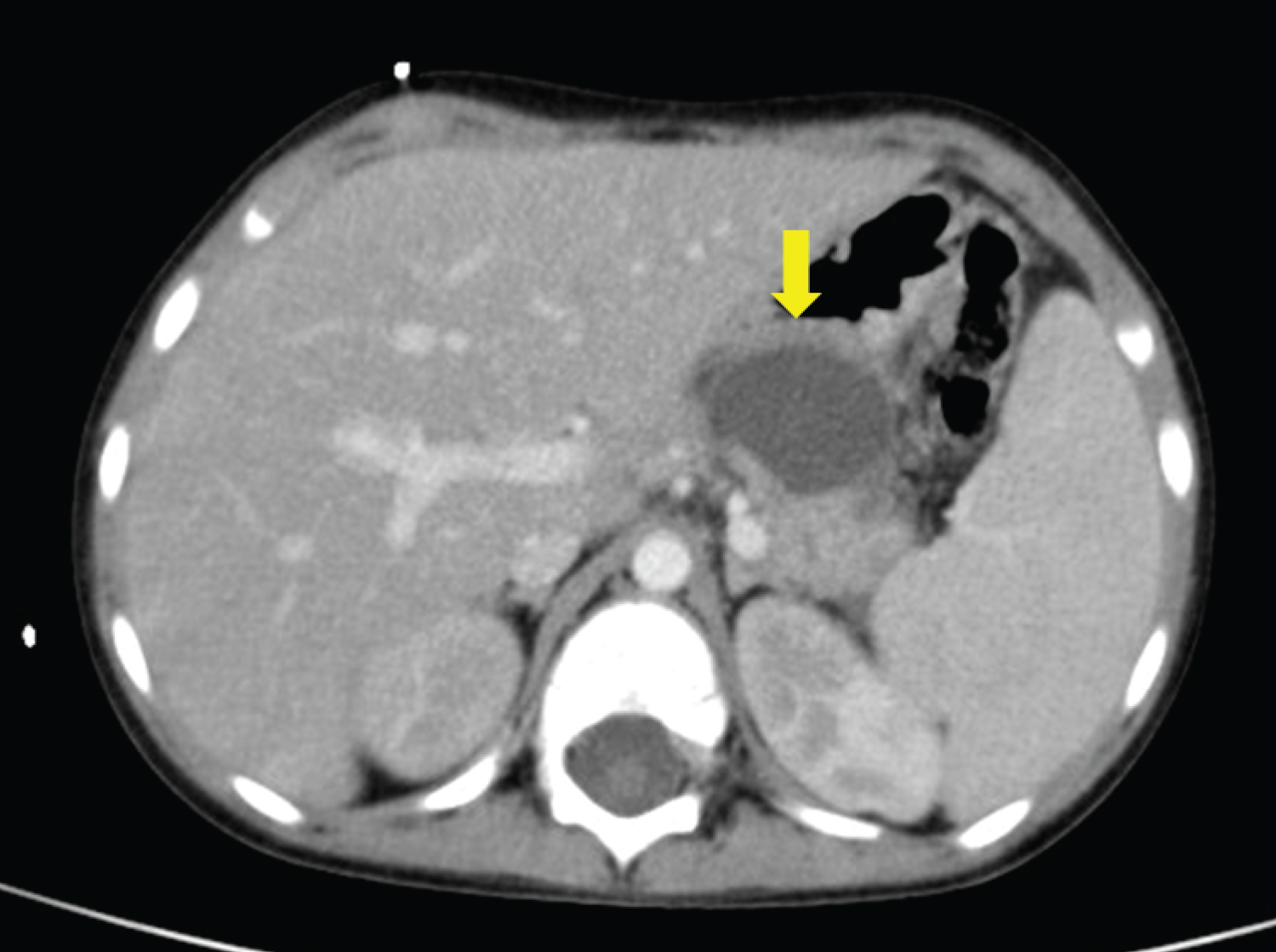 Figure 3: Abdomen CT: evidence of a 3 × 2 cm hypodense lesion in the pancreatic body, indicative of pseudocyst.
View Figure 3
Figure 3: Abdomen CT: evidence of a 3 × 2 cm hypodense lesion in the pancreatic body, indicative of pseudocyst.
View Figure 3
The boy was transferred back to our Unit. Enteral feeding was progressively resumed, with no evidence of steatorrhea; parenteral nutrition was stopped after sixteen days, and also octreotide was discontinued. He was discharged and returned home, after the complete resolution of the symptoms, on the 18 th day of hospitalization. Antileukemic treatment was immediately recommenced and PEG-asparaginase was no longer administered. The child is currently in complete remission during maintenance treatment.
Pancreatitis is one of the most frequent adverse reactions to asparaginase and is a common reason for early drug termination. The pathophysiology of AAP is still unknown; the systemic depletion of asparagine and the subsequent reduction of protein synthesis in the pancreas, which is an organ with a high protein turnover, are considered a plausible pathogenic mechanism [3]. Risk factors for the development of AAP are genetic predisposition [8,9], older age and higher asparaginase dose [10,11]. A consensus definition of AAP across pediatric ALL protocols was recently established by the toxicity working group of the Ponte di Legno Working Group (PTWG) [4]. AAP is defined by at least two of the following features: abdominal pain strongly suggestive of pancreatitis, serum lipase or amylase levels three or more times the upper normal limit, characteristic imaging findings of pancreatitis. Different grades of severity have been recognized by PTWG: grade 1, mild, when symptoms and/or enzyme elevations last less than 72 hours; grade 2, severe, when symptoms and/or enzyme elevations last more than 72 hours, or in case of hemorrhagic pancreatitis, pancreatic abscess, or cyst; grade 3, death from pancreatitis [4]. Another severity grading scale, used for various adverse events in cancer therapy, is the CTCAE (Common Terminology Criteria for Adverse Events); for pancreatitis, there are four grades of severity: grade 2, enzyme elevation or radiologic findings only; grade 3, severe pain, vomiting, and medical intervention indicated (analgesia, nutritional support); grade 4, life-threatening consequences and urgent intervention indicated; grade 5, death. Based on morphological features, acute pancreatitis can be divided into two subtypes: interstitial edematous pancreatitis, characterized by inflammation of the pancreas and peripancreatic tissues without recognizable tissue necrosis, and necrotizing pancreatitis, in which inflammation is associated with pancreatic parenchymal necrosis and/or peripancreatic necrosis; in abdominal imaging, the lack of contrast enhancement of pancreatic parenchyma is indicative of necrosis [1].
We described a case of a severe pediatric necrotizing AAP, grade 2 according to PTWG and grade 3-4 according to CTCAE. Severe forms of AAP are not uncommon: in Samarasinghe, et al., the CTCAE grade 4 of pancreatitis was observed in 27% of cases [13]. In a large study, involving about 465 patients with AAP from several ALL-trial groups, 335 of 374 with available data (90%) developed a severe form of AAP according to PTWG guidelines; the most frequent complications were assisted mechanical ventilation, the need for acute insulin therapy, and pseudocysts. Nevertheless, the number of deaths due to AAP was not elevated (2%) [14].
The management of AAP is generally symptomatic, and includes fasting, intravenous hydration or parenteral nutrition, and pain control with analgesics; also, broad-spectrum antibiotics are used in all cases of AAP in view of the immunosuppression status of children undergoing chemotherapy [3], although the current recommendation is not to administer antibiotics without evidence of infected pancreatic necrosis [15]. Octreotide is a somatostatin analog that inhibits secretion of pancreatic enzymes, reducing pancreatic inflammation. Its efficacy has been evaluated in adult patients with severe acute pancreatitis, demonstrating a decreased rate of systemic complications (sepsis and acute respiratory distress syndrome), hospital stays and mortality [16]. There are no large studies about the use of octreotide in pediatric patients with AAP. In literature, we found only a few pertinent articles [6,17-23]. Including our patient, we identified 13 children (Table 1). Six patients were affected by necrotizing AAP [19-23]; three cases had edematous pancreatitis [17,18], while in 4/13 the type of pancreatitis was not reported [6]. The median age was 8 years; only three patients (23%) were aged 3 years or less at pancreatitis onset. The majority of cases (85%) occurred during the induction phase of ALL treatment; eleven children received L-asparaginase, while PEG-asparaginase was administered in the other two. The median time from the last dose of asparaginase and AAP onset was 5 days (range 1-13 days). The most common complication of the entire cohort was hyperglycemia requiring insulin therapy in 6 patients (46%): in 3 cases, insulin was discontinued after the resolution of pancreatitis [18,23], in 1 child, it was stopped after one year [20], and in 2 patients, diabetes persisted despite recovering from acute pancreatitis [6]. Other reported complications were peripancreatic fluid collection and/or pseudocysts in 5/13 patients (38%) [19,20,22,23], one of them treated with percutaneous drainage [20], and another one who underwent surgical intervention for fat necrosis of the transverse colon [22]: both these two surgically treated patients presented necrotizing AAP. Confirming the severity of this subtype of pancreatitis, 5/6 patients (83%) suffering from necrotizing AAP developed acute complications. No complications were observed in 4 (31%) patients in the cohort of 13. Detailed information about octreotide administration is available in 10/13 patients: in the majority of them (80%), octreotide was used as a continuous intravenous infusion, with an extremely variable dose from 0.2 to 2 µg/kg/hour; the median duration treatment was 7 days (range 4-44 days). There were no side effects associated with the use of octreotide, except for one patient who developed generalized tonic-clonic seizures, a rare event reported in less than 1% of patients [22]. The outcome was favorable in all patients, including those with necrotizing pancreatitis. No case of exocrine pancreatic insufficiency or death was observed.
Table 1: Children with AAP treated with octreotide. View Table 1
These findings are quite different from what was observed in the 4 reported pediatric patients with necrotizing AAP [5,24,25] who did not receive octreotide (Table 2). All of them developed both acute systemic and local complications; furthermore, one patient died [24] and severe late manifestations, such as diabetes and exocrine insufficiency, were reported in another patient. Thus, comparing these two groups of patients affected by necrotizing AAP (the former treated with and the latter treated without octreotide), even though the total number is small, it seems that octreotide can be beneficial in children affected by necrotizing AAP.
Table 2: Cases of pediatric necrotizing AAP treated without octreotide, reported in literature. View Table 2
After the occurrence of AAP, among the 13 patients treated with octreotide, asparaginase was discontinued in 8 children (61%), and in 3 patients, asparaginase formulation was changed with PEG-asparaginase (2/3) or Erwinia asparaginase (1/3), while for the 2 remaining children, no information was reported. Re-introduction of asparaginase after an episode of AAP is still a matter of debate. Asparaginase is a crucial drug in ALL treatment and its discontinuation due to toxicity has been related to decrease disease-free survival [26]. A recent study revealed that the risk of a second AAP was not associated with the severity of the first episode and a second AAP did not involve an increased risk of complications [14]; for this reason, re-exposure to asparaginase should be determined mainly by the anticipated need of asparaginase for its antileukemic efficacy [14]. For patients who would benefit from re-administration of asparaginase for cancer treatment, the prophylactic use of octreotide should be taken into account, as it has demonstrated to be effective in preventing recurrent pancreatitis [27,28].
In conclusion, we have reported a case of necrotizing pancreatitis related to asparaginase, characterized by peripancreatic fluid collections, pseudocyst, peritoneal and pleural effusions, and transient hyperglycemia needing insulin therapy. The recognition of a case of pancreatitis with necrotizing features seems to be significant, as the risk of acute and chronic complications increases markedly when necrosis is present [5]. Octreotide seems to be an effective drug in the treatment of AAP, with an acceptable safety profile. This review has shown that in patients who received octreotide, no acute systemic complications were observed, and local short-term complications usually improved without surgical intervention. Furthermore, octreotide may be beneficial because it could accelerate the resolution of AAP, allowing for earlier resumption of chemotherapy treatment. Further studies are needed to verify the efficacy of octreotide in the treatment of AAP and, also, in the prevention of recurrent pancreatitis in children with ALL.
The authors wish to thank Prof. Matthew Furfine for revising the manuscript, and the Parents’ Association “A.S.L.T.I. - Liberi di crescere” Onlus for financial support.
The authors report no conflict of interest.
Written informed consent was obtained from the patient and his parents for publication of this case report.
The data that support the findings of this study are available on request from the corresponding author. The data are not available for publication due to privacy or ethical restrictions.
This research received no external funding.
All the Authors equally contributed to the manuscript, and have reviewed and agreed upon the paper content.