Renal cell carcinoma (RCC) is the most common malignant neoplasm of the kidney and constitutes about 2-3% of all cancers worldwide. In 30% of tumours, patients present with metastasis. The prognosis of patients with RCC varies according to the stage and histological grade [1].
In 2016, the new WHO classification has classified t(6;11) renal cell carcinoma and Xp11.2 translocation/TFE3 gene fusion-related renal cell carcinoma into MIT family translocation renal cell carcinoma [2,3]. Now classification system has incorporated electron microscopy, immunohistochemistry, cytogenetic, and molecular diagnostic techniques. Some tumors such as ALK rearrangement-associated RCC, MiT family translocation renal carcinomas, SDH-deficient renal cancer or FH-deficient RCC, are defined by their molecular characteristics [4]. This type of RCC is generally characterized by a range of translocations on chromosome Xp11.2 leading to a gene fusion between TFE3 and at least 6 possible partners [5].
Children are more affected by this subtype than adults, accounts for 20-40% of pediatric RCC and 1-1.6% of RCC in adults.
The diagnosis of Xp11.2 translocation RCC is based on fluorescent in situ hybridization (FISH) which is gold standard rather than histological characteristics and imaging examination [6]. However, diagnosis can be made in Immunohistochemistry.
Here, we discussed 3 cases of Mit translocation RCC based on clinical histological and immunohistochemistry.
A 42-year-old female a known case of left non-functioning kidney secondary to PUJ obstruction and hepatic cysts has been admitted at hospital for further management. On physical examination and systemic examination patient was normal. Intraoperative findings were hydronephrotic kidney with brownish coloured fluid with single artery and single vein.
Gross: Received a Left nephrectomy specimen measuring 12.5 × 12 × 4 cm. External surface is congested cut section shows dilated pelvic-calyceal. Multiple papillae excrescences seen each measuring 1.1 to 1.5 in greatest dimension. Cut section of papillary excrescences is grey brown to grey white. Ureter measures 0.5 cm in length.
Microscopy: Section revealed tumor arranged in papillary and focal tubular structures. Cells show centrally placed vesicular nuclei with prominent nucleolus, granular chromatin and eosinophilic cytoplasm Occasional mitosis seen. Tumor is limited to kidney. Gerotas fascia, renal capsule and renal sinus are free of tumor.
Multifocal in nature, ISUP grade 2 with papillary architectural suggest possibility of papillary renal cell carcinoma on H&E. IHC shows AMACR, Vimentin and RCC positivity. CD10, CK7, CD117 were negative. Ki67 [3%] and TFE3 Immunoreactive, Score 3+ in lesional cells and reported as MiT family translocation renal cell carcinoma [m pT1a] (Figure 1).
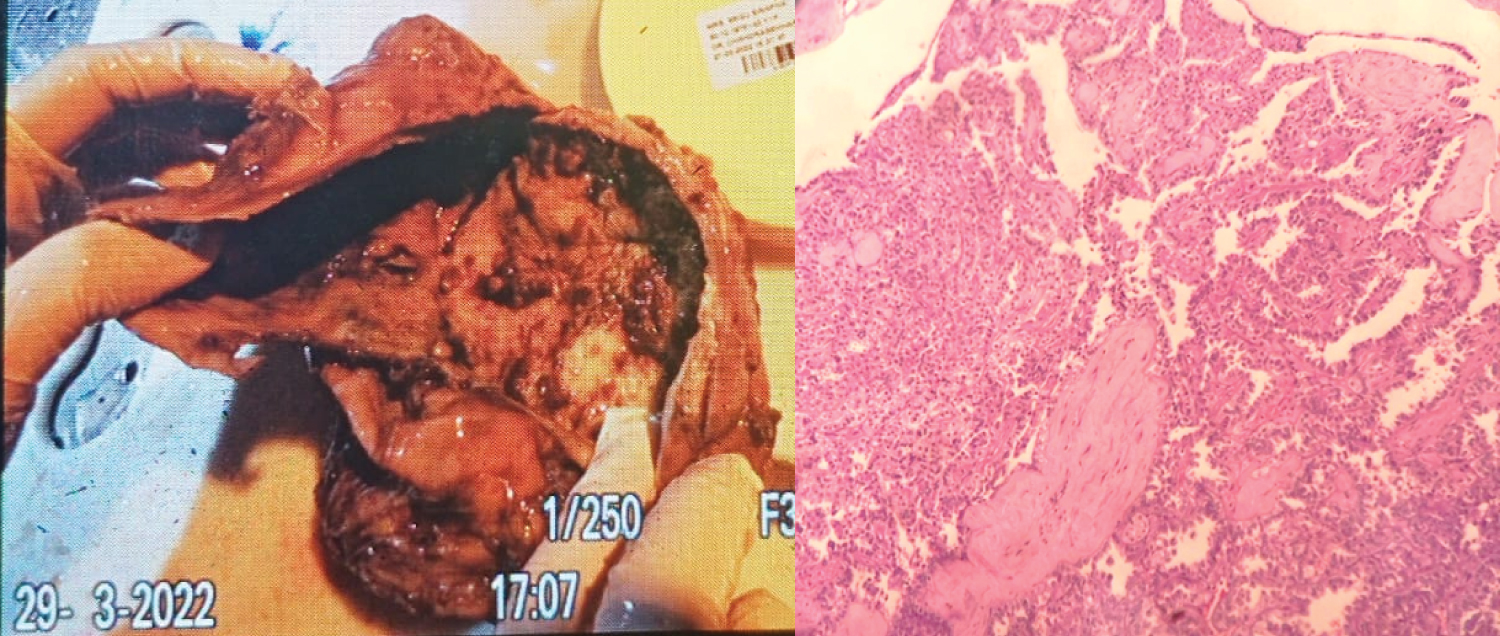 Figure 1: Gross: 1a Showing multiple excrescences; H & E: 1b Papillary and focal tubular pattern. Cell shows centrally placed nuclei, granular.
View Figure 1
Figure 1: Gross: 1a Showing multiple excrescences; H & E: 1b Papillary and focal tubular pattern. Cell shows centrally placed nuclei, granular.
View Figure 1
A 5-year-old female presented with haematuria and weight loss since 6 months associated with low grade fever on and off and urge incontinence.
On physical examination and systemic examination patient was normal.
CT renal angiography was done which shows well defined mixed density heterogeneously enhancing rounded right renal mass lower pole: neoplastic aetiology. USG abdomen shows heterogenous hyperechoic mass at lower pole of right kidney measuring 24 × 25 mm. Renal core biopsy done. On H&E possibility of MiT family translocation renal cell carcinoma and metanephric adenoma. IHC shows PAX8, CD10, Vimentin, AMACR, Ki67 [8%] and TFE [3 +] positivity gives final diagnosis of MiT family translocation Renal cell carcinoma.
After that Right retroperitoneoscopic radical nephrectomy done.
Gross: Received nephrectomy specimen 8 × 4 × 2 cm ureter measures 4 cm. A well circumscribed tumor at lower pole measuring 3 × 2.8 × 1 cm at a distance of 6 cm from ureteric cut end.
Microscopy: Section show tumor arranged in acinar and papillary pattern. Cells having vesicular nuclei, prominent nucleoli, granular chromatin and abundant clear cytoplasm. No sarcomatoid and Rhabdoid features seen. Focal necrosis present. Tumor confined to kidney. Gerotas fascia, renal sinus are free from tumor. Diagnosis of Mit family translocation RCC pT1a was given (Figure 2).
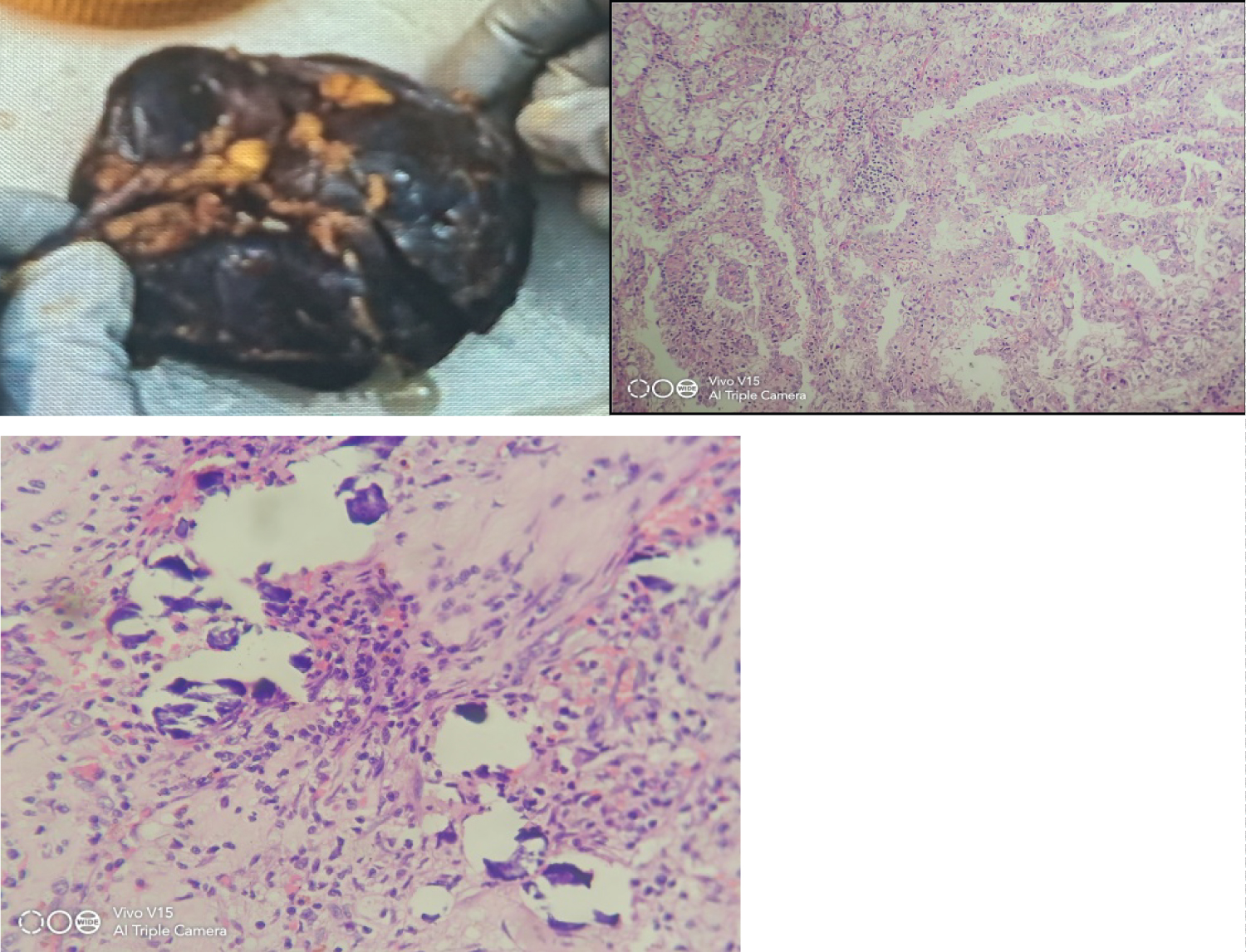 Figure 2: Gross: 2a, H & E 2b, 2c: Showing papillary pattern with calcification.
View Figure 2
Figure 2: Gross: 2a, H & E 2b, 2c: Showing papillary pattern with calcification.
View Figure 2
A 60-year-old male presented, on physical examination and systemic examination patient was normal. USG abdomen shows large hetrogenous exophytic mass measuring 5.5 × 5.2 cm in interpolar region. Intraoperative shows distortion of renal cortex with intact Gerotas fasica.
Gross: Received Left nephrectomy specimen measuring 8.8 × 8 × 6 cm. Tumor seen at mid pole measuring 5.5 × 4.5 × 3 cm. cut section grey white to grey brown at a distance of 4 cm from ureteric cut margin.
Microscopy: Section shows tumor arranged in alveolar, acinar and dilated acinar structures and focal papillary architecture. Cells show centrally placed vesicular nuclei to eosinophilic cytoplasm. No sarcomatoid, Rhabdoid differentiation and necrosis seen. ISUP grade 2. Tumor is limited to kidney. All margins free from tumor. Possibility of papillary, clear cell and Mit RCC considered on H&E. IHC shows CD10 and Vimentin positive, AMACR, CD117, CK7 were negative, and Ki67 15% and TFE3 score 3+ in lesional cells. Final diagnosis of MiT translocation renal cell carcinoma with staging pT1b (Figure 3, Figure 4 and Table 1).
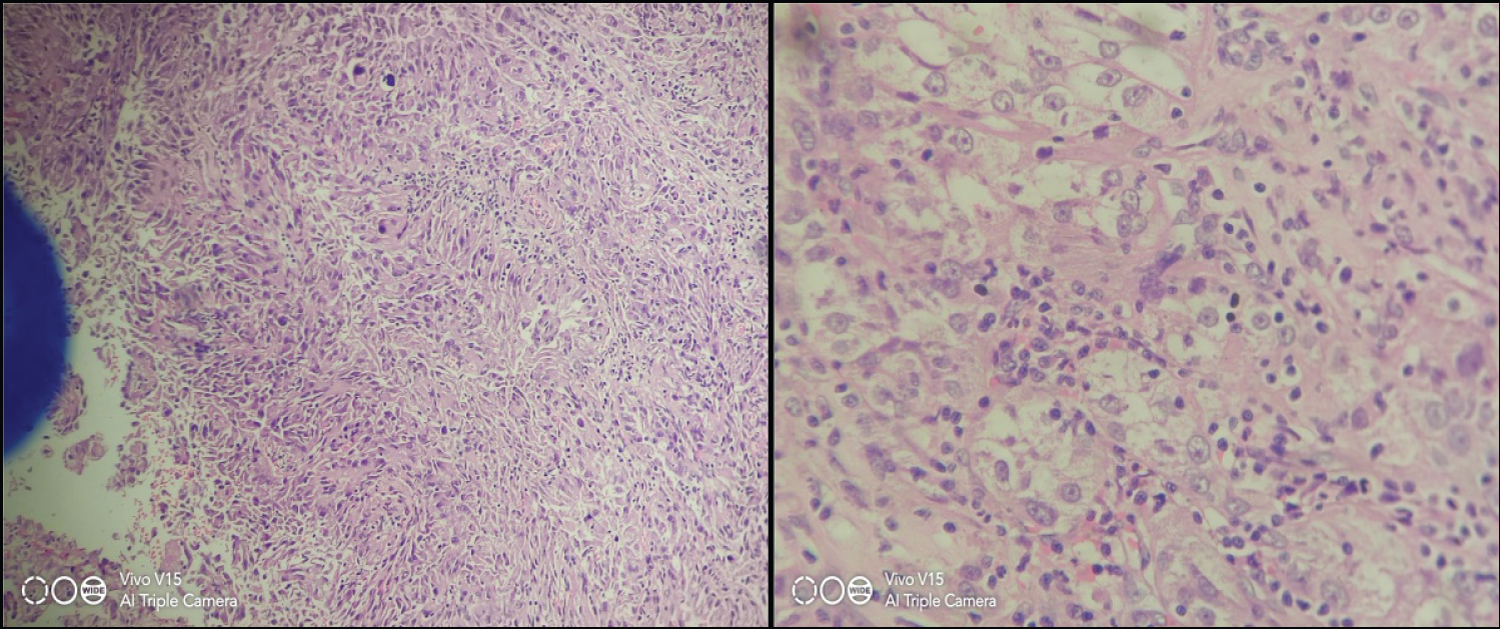 Figure 3: H & E microscopy [3a n 3b]: Showing acinar pattern with marked nuclear pleomorphism.
View Figure 3
Figure 3: H & E microscopy [3a n 3b]: Showing acinar pattern with marked nuclear pleomorphism.
View Figure 3
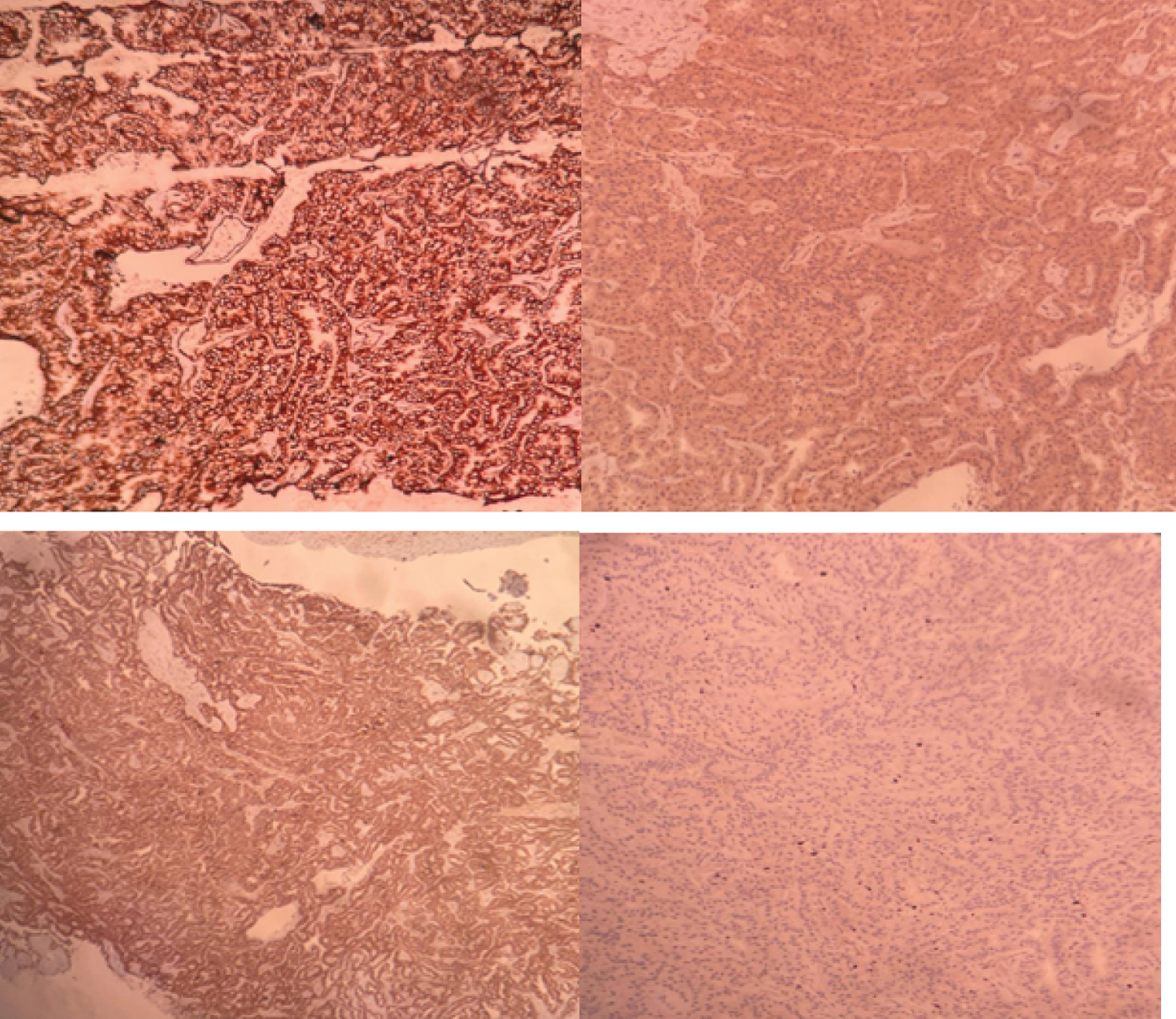 Figure 4: IHC: A) Vimentin; B) AMACR; C) RCC; D) Ki-67%.
View Figure 4
Figure 4: IHC: A) Vimentin; B) AMACR; C) RCC; D) Ki-67%.
View Figure 4
Table 1: Case study of patients. View Table 1
Xp11.2 translocation RCC with TFE3 gene fusion is a rare tumor, and adult Xp11.2 translocation RCC has a poorer prognosis compared with its pediatric counterpart. The aim of the present study was to report three rare cases in three month involving 2 elderly i.e. 1 male and 1 female patient and 1 pediatric patient. With Xp11.2 translocation RCC with TFE3 gene fusion and review the relevant literature to help elucidate the characteristics of this rare type of cancer.
XP11.2 translocation RCC accounts for 20-40% of pediatric RCC and only 1-1.6% of adult RCC. This subtype of RCC in adults requires special attention and more intensive studies for its rarity, aggressiveness in nature, and possible different treatment options (e.g., mTOR inhibitors or VEGF-targeted agents) [7]. The different gene fusions may be associated with different clinical and morphological characteristics [8]. So it´s know that Xp11 translocation renal cell carcinoma is cytogenetically characterized by chromosomal translocations involving breakpoints in the TFE3 gene, which maps to the Xp11.2 locus.
The gross features of Xp11.2 translocation RCC are similar to those of conventional clear cell RCC. Macroscopically, the cut surfaces of the tumors are greyish-yellow in colour and exhibit haemorrhaging and necrosis [9]. Microscopically, MiTF translocation RCC shows a mixed papillary, solid, and alveolar growth pattern. Furthermore, tumor cells are enlarged with voluminous cytoplasm, distinct cell borders, and have a clear or eosinophilic appearance. Due to various histological presentations, translocation RCC can resemble other forms of RCCs such as clear cell RCC, multilocular cystic RCC, papillary RCC, urothelial carcinoma, collecting duct carcinoma, mucinous tubular carcinoma, or Epitheloid angiomyolipoma [10].
Due to the translocations lead to over expression of TFE3 protein; detection of TFE3 protein by IHC assay is currently the most commonly used diagnostic technique in clinical practice [11]. The immunohistochemistry results were scored based on both the staining intensity (none: 0; weak: 1; moderate: 2; and strong: 3) and extent (< 11%: 1; 11-50%: 2; 51-75%: 3; and > 75%: 4) using previously a published scoring method. The intensity and extent scores were then multiplied to yield final scores that ranged from 0 to 12; scores ≥ 3 were considered positive [12].
The average survival time of the TFE3 associated RCC in adults can reach 2 years, while the average survival time of children is 6.3 years [13]. Furthermore, it has been reported that ~15% of the adult patients with Xp11.2 translocation RCC had a history of chemotherapy; thus, the occurrence of this cancer may be correlated with chemotherapy [9].
The treatment for Xp11.2 RCC varies; however, there has been no established effective treatment to date. Surgery is the main treatment similar to that for conventional RCC. For localized Xp11.2 RCC with positive regional lymph nodes, surgery is the optimal treatment [1]. For patients with haematogenous metastases, the current options are immunotherapy using cytokines, such as interleukin 2 (IL-2) and interferon alpha (IFNα), and multikinase inhibitors. Malouf, et al. analysed the benefit of targeted therapy (VEGFR targeted agents and/or mTOR inhibitors) in patients with Xp11 translocation/TFE3 fusion gene metastatic RCC and found better response in terms of median progression-free survival (PFS) as compared to PFS of 2 months when receiving a cytokine-based regimen [14].
The grim clinical outcome associated with Xp11.2 RCC warrants early detection, accurate diagnosis, and close follows up. Till recently no surveillance algorithm for Xp11 TRCC after radical nephrectomy was developed.
Xp11.2 translocation renal cell carcinoma is rare and Number of cases is very less in literature. The exact biologic behaviour, treatment modalities remain uncertain. So awareness among urologist, pathologist and oncologist is necessary to identify more cases of this phenotype in future. Early diagnosis and treatment in early stage have good prognosis.