Background: Hidradenocarcinoma (HAC) is an exceedingly rare and aggressive malignant tumor of the adnexal eccrine and apocrine sweat gland. Due to its low incidence rate, nonspecific clinical presentation, and similar histopathological features to benign hidradenoma (HA), HAC is often misdiagnosed as its benign counterpart. Histologically, even the absence of cellular atypia does not rule out malignancy. Oftentimes HAC is retrospectively diagnosed when recurrence or metastatic lesions arise post-surgical excision.
Methods: We present a case of HAC of the scalp of a 64-year-old female with initial biopsy results resembling HA.
Results: The patient's HAC was successfully treated with two stages of Mohs micrographic surgery (MMS) with clear tumor margins.
Conclusion: There is a current lack of consensus in treatment and management of HAC and HA. MMS should be the preferred treatment for both HAC and HA, as it allows for systematic evaluation of 100% of the tumor margins. The current literature and as evidenced by this case report, has proven efficacy and beneficial patient outcomes with the implementation of MMS.
Hidradenocarcinoma, Mohs micrographic surgery, Hidradenoma
HAC: Hidradenocarcinoma; HA: Hidradenoma; MMS: Mohs micrographic surgery
HAC is a rare malignant adnexal neoplasm of eccrine and apocrine origin [1]. HAC commonly manifests on the head, neck, and face, and presents most commonly during the fifth to seventh decades of life [2]. Due to almost indistinguishable histological differences from its benign counterpart, HA, HAC is particularly challenging to diagnose. The typical clinical presentation of HAC is a solitary, firm, subcutaneous nodule or plaque. The lesion may occasionally be erythematous and ulcerated. Thus, the non-descript clinical findings can easily be dangerously misdiagnosed as some of the more common benign adnexal etiologies such as pilar or infundibular cysts [2]. Due to the exceedingly low incidence rate of HAC, few literature reports in cases and management of HAC have been published. Currently, no consensus treatment for HAC exists. Numerous synonyms for this diagnosis include clear cell-eccrine carcinoma, malignant clear cell HAC, primary mucoepidermoid cutaneous carcinoma, solid cystic adenocarcinoma, malignant clear-cell myoepithelioma, and malignant acrospiroma- further driving disorganization in management in the current literature [3,4]. Here, we present a case of HAC of the scalp in a patient who was subsequently managed with MMS. Consent was obtained from the patient.
The patient is a 64-year old female with a one-year history of a slowly enlarging nodule on her scalp. The lesion was pruritic and it easily bled upon minor trauma. She denied pain or purulent discharge. The patient had a past medical history of hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, cataracts, lung nodules, and latent tuberculosis. For these conditions, she was on carvedilol, losartan, and warfarin. The patient had no personal or family history of skin cancer. She denied any drug allergies. She denied history of tobacco, alcohol, or illicit drug use. Upon review of systems, she denied fevers, chills, night sweats, or weight loss. On physical examination of the vertex scalp, there was a 12 × 12 mm friable exophytic nodule with a reddish appearance (Figure 1A). There was no cervical, occipital, supraclavicular, or axillary lymphadenopathy. A PET/CT examination of the skull to mid-thigh level demonstrated no abnormal uptake.
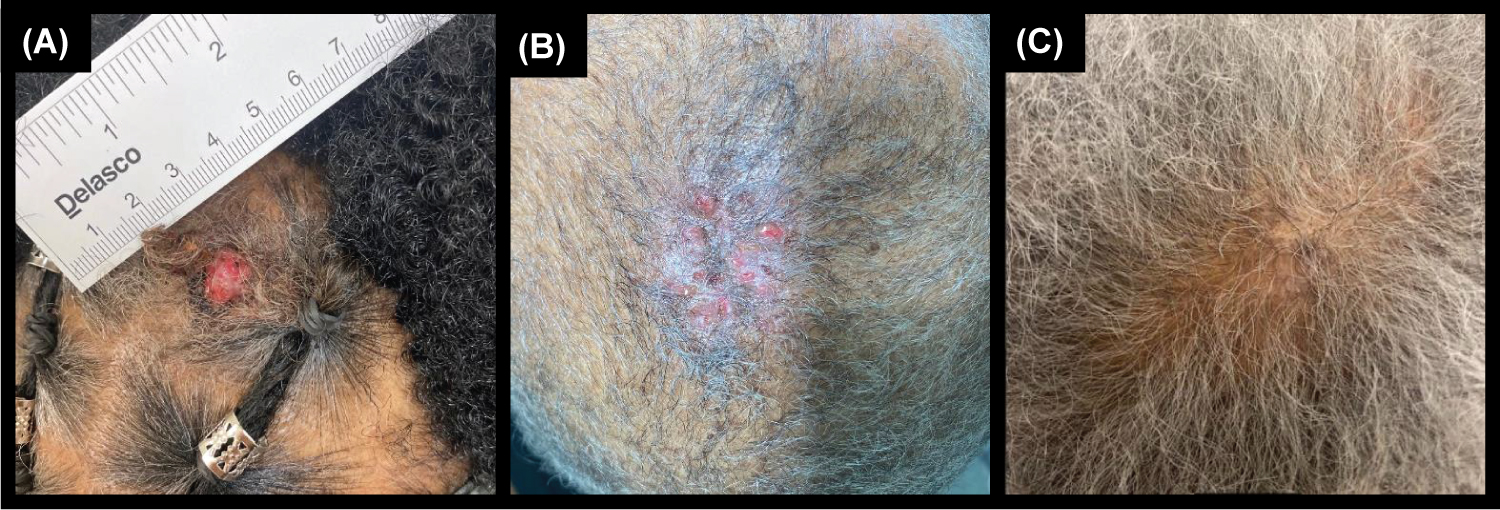 Figure 1: A) Hidradenocarcinoma of the scalp prior to excision. B) Two week follow-up visit after Mohs micrographic surgery (MMS). C) Three month follow-up visit after MMS.
View Figure 1
Figure 1: A) Hidradenocarcinoma of the scalp prior to excision. B) Two week follow-up visit after Mohs micrographic surgery (MMS). C) Three month follow-up visit after MMS.
View Figure 1
After informed consent was obtained from the patient, a shave biopsy of the lesion was performed. On histopathology (Figure 2), although overall findings mostly represented an atypical clear cell HA, the presence of nuclear pleomorphism, scattered mitotic figures, focal invasion, and focal tumor necrosis indicated the diagnosis of HAC. Sections demonstrated a nodulocystic proliferation of epithelial cells with cellular polymorphism, including clear cells, secretory cells, basaloid cells, and focal mucinous goblet-like cells. The tumor cells showed focal nuclear pleomorphism and scattered mitotic figures. Luminal formation was present as well as collections of eosinophilic basement membrane material within the tumor. Focal ulceration and invasion was also identified at the periphery of the tumor. The patient underwent MMS after informed consent was obtained. The procedure required a total of two stages for complete clearance of tumor and any cellular atypia. The surgical defect was reconstructed with a bilateral advancement flap. Sutures were removed two weeks following the procedure with proper healing (Figure 1B). The patient was seen for total body skin examination after three months with no concerning lesions and no recurrence noted (Figure 1C).
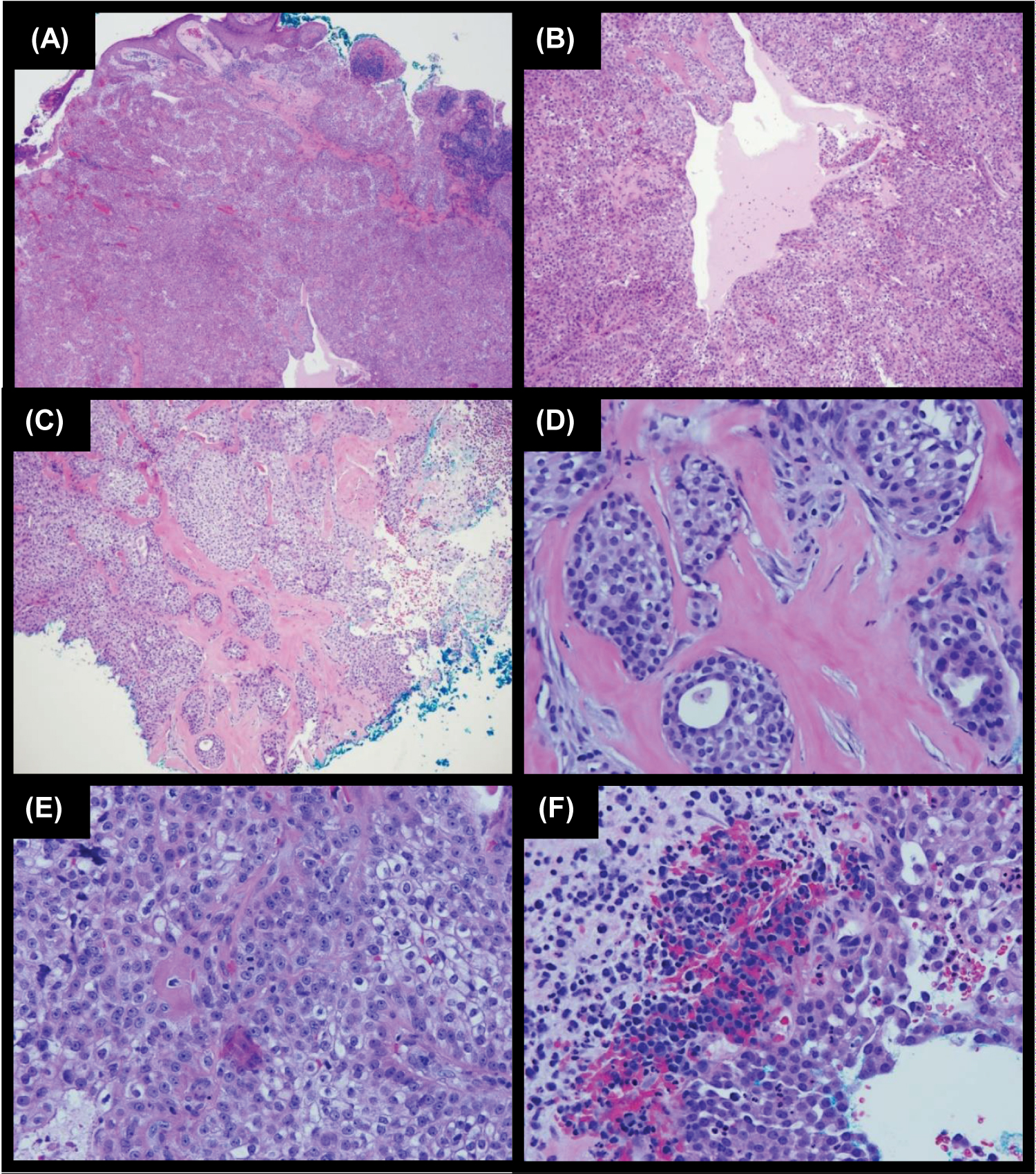 Figure 2: H&E stains of hidradenocarcinoma specimen of our patient. A) 4X: Section shows focal ulcer. The dermis contains lobules of epithelioid cells. B) 10X: The tumor cells show round nuclei and prominent clear cytoplasm. There are collections of eosinophilic basement membrane material within the tumor. There are microtubules and cystic changes in the tumor. C) 10X: There is focal invasion identified at the periphery of the tumor. D) 40X Focal invasion on a higher power. E) 40X: The tumor cells show focal nuclear pleomorphism and scattered mitotic figures. F) 40X: There is focal tumor necrosis.
View Figure 2
Figure 2: H&E stains of hidradenocarcinoma specimen of our patient. A) 4X: Section shows focal ulcer. The dermis contains lobules of epithelioid cells. B) 10X: The tumor cells show round nuclei and prominent clear cytoplasm. There are collections of eosinophilic basement membrane material within the tumor. There are microtubules and cystic changes in the tumor. C) 10X: There is focal invasion identified at the periphery of the tumor. D) 40X Focal invasion on a higher power. E) 40X: The tumor cells show focal nuclear pleomorphism and scattered mitotic figures. F) 40X: There is focal tumor necrosis.
View Figure 2
HAC is a primary, conventionally de-novo, eccrine carcinoma that comprises less than 0.01% of all skin cancers and less than 0.001% of all tumors [5-7]. This pathology typically manifests in females during ages 50 to 70 years old, with the average age of onset being in the sixth decade of life [8]. Though typical manifestation occurs during adulthood, incidences of HAC during childhood have been reported as well [3,9]. HAC rarely arises from a pre-existing benign HA [10]. The low incidence rate of this pathology leads to a scarce amount of literature regarding management of HAC. As of 2021, approximately only about 150 cases have been cited.
Clinically, HAC commonly presents on the head and neck, especially on the face [2,11]. Further in its progression, it can present in distal extremities [8]. With high metastatic potential, metastasis typically occurs in the lymph nodes in 39% of cases and to visceral organs including lung, pleura, and bone in 28% of cases [1,12,13]. Rarely, it has been reported that HAC may present on the digits, axilla, trunk, abdomen, and in our patient's case, the scalp [7,14]. The lesion presents as a well circumscribed, 1-5 cm fleshy red, gray, or violet, solitary, subcutaneous, firm nodule, or erythematous plaque [2,11]. Telangiectasias or ulceration may also accompany the lesion. The lesion may remain local with slow expansion over a period of months to years and may commonly be asymptomatic [12]. However, pain, erythema, discomfort, and bleeding upon aggravation may also be present. Because HAC lesions lack a distinct clinical appearance, they commonly mimic benign and other malignant adnexal pathologies such as pilar or infundibular cyst, lipoma, fibroma, basal cell carcinoma, hemangioma, lymphangioma, melanoma, merkel cell carcinoma, and squamous cell carcinoma [4]. Thus, it is important that diagnosis of HAC be made histologically instead of clinically.
On histopathological examination, it must be noted that HAC is incredibly challenging to differentiate from the benign HA. As such, this difficulty has been well reported in literature. Lesions which were deemed histologically benign have been retrospectively diagnosed as malignant post histopathologic reexamination following appearance of metastatic lesions or aggressive recurrence after excision [2,15]. HAs are well-circumscribed, non-encapsulated lobular dermal masses with focal or lack of connection to the epidermis. Classically, there is evidence of scattered sweat ducts within tumor nodules and prominent dermal sclerosis with keloidal collagen [9,16]. Cell types found within HAs include squamoid, poroid, and clear cells [9]. Although HAs are primarily nodular, large cystic spaces may be present with encasing eosinophilic homogenous material [17]. Histological features of HAC, which differentiate this pathology from HA, include comedo-like necrosis, lymphovascular invasion, perineural invasion, hemorrhage, nuclear pleomorphism, increased mitotic activity, and atypical mitoses [2,7,15]. Asymmetrical appearance and loss of circumscription may also be observed. Fulfillment of the sole-criteria of tumor cords with peripheral invasion alone warrants diagnosis of HAC [2]. Of note, absence of any of the aforementioned atypia does not rule out malignancy.
The treatment of choice for HA is complete surgical excision to assure that the tumor is truly HA and not HAC lacking cytologic atypia, as such variants may only be recognized by infiltration into surrounding tissue [11]. Traditional histologic methods of determining tumor margins may miss tentacle like projections of tumor cells in unexamined sections, since only a percentage of tumor margin is examined. Because of the rarity of HAC and the variation in presentation, no consensus about the treatment protocol for the management of HAC exists today. Traditionally, HAC has been managed with wide local excision with recurrence and metastatic rates up to 20-50% [1,4,18]. In the past decade, case reports and case series for the implementation of MMS have shown promising results. The literature on MMS for HAC as well as HA [19], however limited, demonstrates efficacy in tumor clearance because 100% of the tumor margins are systematically analyzed. Additionally, MMS methods of tissue preservation and increased repair options are especially necessary, since majority of HAC, lesions are located on the head and neck. Consequently, improved patient outcomes and decreased morbidity are expected [2,18]. A Mayo clinic study demonstrated that in an average of 1.5 stages, MMS led to complete clearance of HAC with zero recurrences and metastases in a 7-year follow-up period [18]. In our patient, two rounds of MMS stages were required for complete clearance of cellular atypia.
Like the treatment of other skin carcinomas, adjuvant therapy is based on grade, margin positivity, angiolymphatic invasion, and metastasis; however, like primary management, no consensus for adjuvant treatment for HAC exists. While some recommend sentinel lymph node biopsy (SLNB) to detect subclinical metastases, the use of SLNB remains questionable [20]. Radiation therapy is used selectively in patients when tumor is unresectable, for improvement in local control if lymph node involvement is evident, or if primary surgery is incomplete [4,12]. Adjuvant chemotherapy may play a role in the metastatic setting. Reports of such agents include 5-fluorouracil, capecitabine, bleomycin, vincristine, cyclophosphamide, doxorubicin, and platins. Targeted therapies currently under investigation include trastuzumab, EGFR inhibitors, PD-1 inhibitors, and PI3K/Akt/mTOR pathways [4,11,12]. Because lymphadenopathy was not present in our patient, and tumor margins were cleared with MMS, adjuvant therapies were not pursued.
Follow-up for patients should occur on a monthly basis to ensure absence of local recurrence, metastasis to lymph node or visceral organ, and/or radiation toxicity. If suspicions are high, radiologic imaging modalities such as x-ray, CT scan, and PET scan may be implemented [12].
HAC is an exceedingly rare and aggressive tumor with high metastatic potential. With the challenge of nonspecific clinical presentation, and histopathology similarities to benign HA, HAC maybe commonly misdiagnosed, further delaying potentially life-saving treatment. It is of greatest importance that malignancy be diagnosed early on in its discovery. While the mainstay of treatment for both HA and HAC is wide local excision, MMS has proven to be far more efficacious. Consequently, Mohs micrographic surgery should be the preferred treatment not only for HAC, but also for HA because it allows for systematic evaluation of 100% of the tumor margin. MMS thereby minimizes potential misdiagnoses of benign causes and improves outcomes and morbidity for a patient with HA and HAC.
Thank you to the University of Illinois at Chicago Dermatology Department.
Authors do not have conflict of interest to declare.
JL wrote the manuscript and drafted figures. CP designed the study. CP, RH, and WL acquired and analyzed the data. VD acquired the data and performed the surgery.