Early diagnosis of tuberculosis is important for therapeutic reasons and to control the spread of infection [1,2]. Culture of M. tuberculosis is the gold standard method for the diagnosis of TB [3]. However, culture is a slow process requiring specialized laboratories and skilled staff. Hence there is a need for a rapid, cheaper and effective technique for the detection of the tubercle bacilli.
A total of 200 clinically suspected cases of tuberculosis were included in the study. All the cytological specimens procured were smeared and stained for both ZN and FL staining. A part of the sample was used for Lowenstein Jensen (LJ) culture. Patients on ATT were excluded from the study.
The maximum cases were in the age group of 21-30 years. In 57% cases, patients were male with M:F ratio of 1.3:1. The sensitivity of FL (95.83%) was more as compared to ZN (91.67%). The difference in the case detection rate was statistically significant with p value 0.001. The average time taken to screen per slide by ZN was more (4.32 mins) as compared to that by FL (2.28 mins), reflecting a time saving by 47%.
FL staining has an upper edge in respect to efficacy, time saving and less observer fatigue. Hence replacement of the age old ZN technique and using FL microscopy may be considered as alternative for diagnosis of tuberculosis.
Ziehl-Neelsen, Fluorescent, Tuberculosis, Culture
As per the World Health Organization (WHO), tubercular infections are presently spreading at the rate of one person per second per million people, with three millions dying from it [1]. Early diagnosis of tuberculosis is important for therapeutic reasons and to control the spread of infection [2]. Culture of M. tuberculosis is the gold standard method for the diagnosis of TB [3]. However, culture is a slow process requiring specialized laboratories and highly skilled staff. In developing countries like India with a high tuberculosis burden and limited number of adequate resources and infrastructure, the diagnosis of Tuberculosis relies mostly on smear microscopy for Acid Fast Bacilli (AFB), however its sensitivity is considered to be low in paucibacillary cases [4].
Although several research groups have investigated the clinical validity and differences in efficacy of various staining methods, the technical and procedural factors can influence the sensitivity of each staining technique. Moreover, very little previous literature related to the comparison of Auramine-o stained smears under fluorescent microscopy with ZN stain for detection of tubercle bacilli in cytological specimens is available till date.
Hence, in view of above perspective, the present comparative study has been designed to assess the efficacy of Ziehl-Neelsen staining method versus fluorescent staining in the detection of mycobacterium from various cytological specimens of suspected cases of tuberculosis.
The study was conducted in the Cytology unit of Department of Pathology in collaboration with Department of Microbiology over a period of 12 months after obtaining approval from the institutional ethical committee.
A total of 200 consecutive suspected cases of tuberculosis were included in the study.
FNAC of lymph node, irrespective of age and gender with suspicious clinical history or radiological evidence were included in the study along with other body fluids.
Pre-diagnosed cases already on anti-tubercular therapy.
Ziehl-Neelsen and Auramine-o staining (Sigma-Aldrich) was done as per Standard Operating Protocol. Grading of the smears was done as per the RNTCP guidelines. Culture on L-J media was done to compare the two staining procedures.
Interpretation and analysis of obtained results was carried out using software SPSS version 20 and appropriate statistical test were employed.
In the present study, 57% cases (n = 114) were males and 43% (n = 86) were females with male to female ratio of 1.3:1. Maximum number of cases (n = 52/200; 26%) were in the age group of 21-30 years (Figure 1).
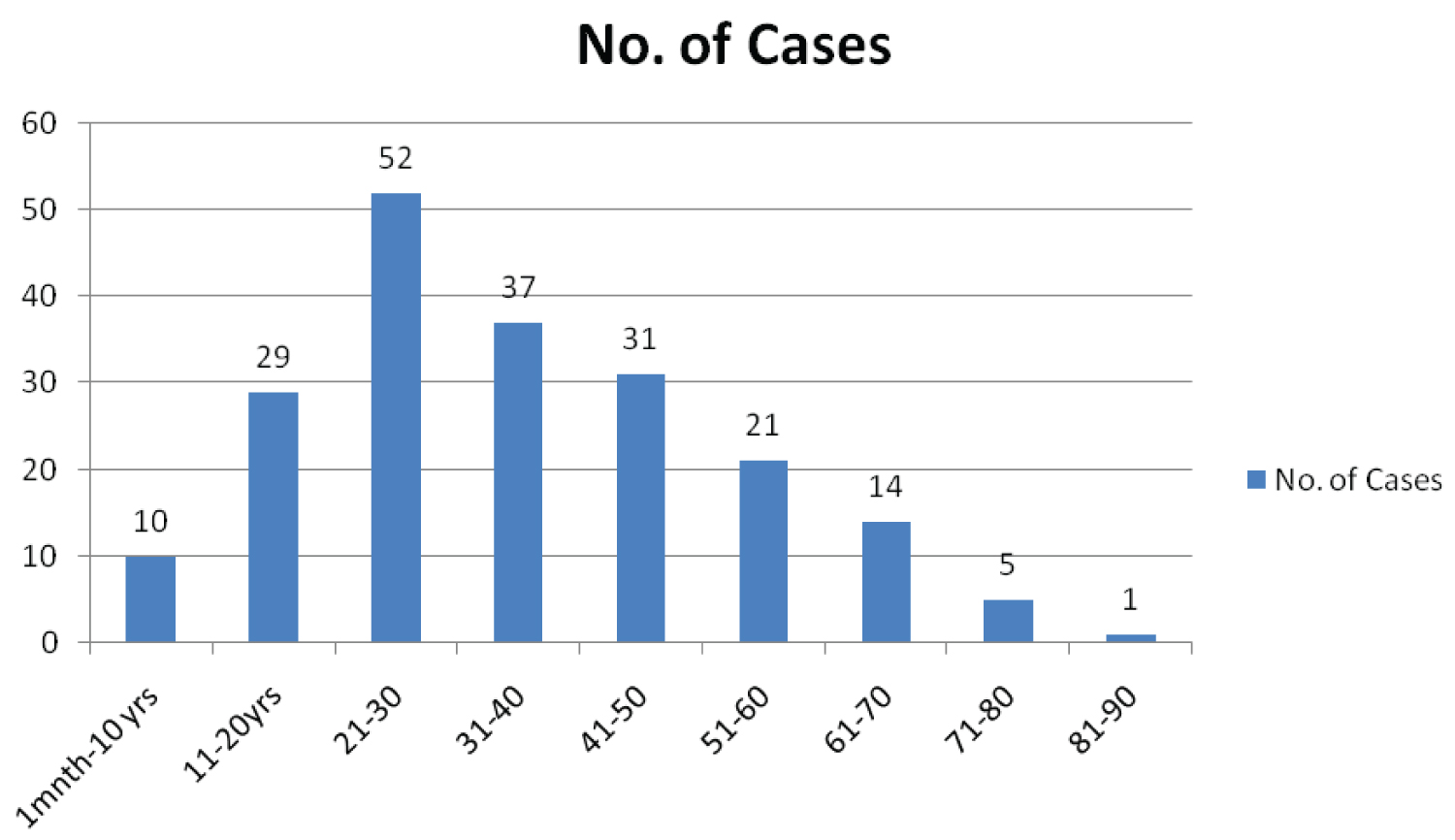 Figure 1: Distribution of cases according to age (n = 200).
View Figure 1
Figure 1: Distribution of cases according to age (n = 200).
View Figure 1
The most common presenting complaint of the cases was fever (n = 177/200; 88.5%) followed by malaise (n = 115; 57.5%) and cough (n = 109; 54.5%). Maximum number of cases were lymph node aspirates (n = 79; 39.5%) followed by pleural fluid (n = 45; 22.5%) and pus (n = 44; 22%). There was one case each of Endotracheal tube secretions and Trans Bronchial Needle Aspiration.
Cervical lymph node was the most frequently aspirated lymph node (n = 71; 71%) followed by supraclavicular lymph node (n = 11; 11%). The maximum number of positive cases was seen in Cervical lymph node (n = 13), followed by two cases each of breast lump and vertebral aspirate.
Of the 200 samples processed, a total of 25 cases were positive either on ZN (Figure 2) or FL (Figure 3), majority of the cases (92%, n = 23) were paucibacillary. The multibacillary cases were diagnosed equally by both the staining methods, however the only true positive case diagnosed in addition on FL was paucibacillary (Table 1).
Table 1: Test results. View Table 1
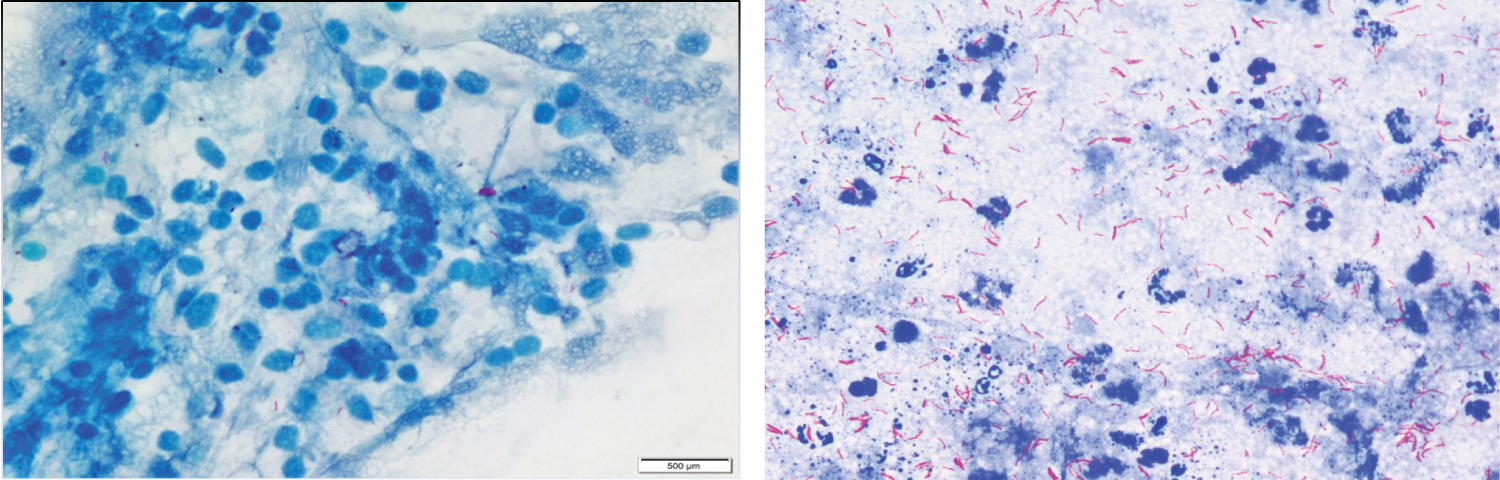 Figure 2: (A) Paucibacillary case on ZN staining at 100x (oil immersion view; (B) Multibacillary case on ZN staining at 100x (oil immersion view).
View Figure 2
Figure 2: (A) Paucibacillary case on ZN staining at 100x (oil immersion view; (B) Multibacillary case on ZN staining at 100x (oil immersion view).
View Figure 2
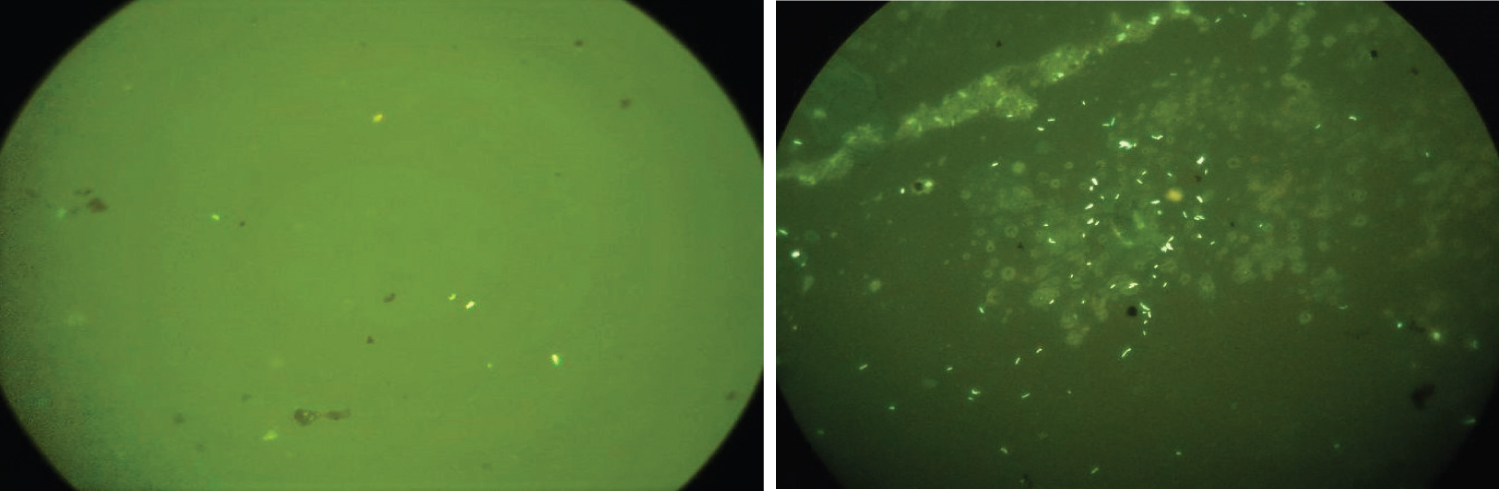 Figure 3: (A) Paucibacillary case on FL staining at 40x (high power view); (B) Multibacillary case on FL staining at 40x (high power view).
View Figure 3
Figure 3: (A) Paucibacillary case on FL staining at 40x (high power view); (B) Multibacillary case on FL staining at 40x (high power view).
View Figure 3
The isolation rate by LJ culture was 12% (24/200) (Figure 4). The difference in case detection rates by ZN and FL is statistically significant with p-value 0.001 by Z-test.
 Figure 4: Lowenstein-Jensen medium slant showing buff coloured and rough colonies of the tubercle bacilli.
View Figure 4
Figure 4: Lowenstein-Jensen medium slant showing buff coloured and rough colonies of the tubercle bacilli.
View Figure 4
Smear microscopy remains the crux for diagnosis of TB. However, the search for rapid and efficient staining methods continues.
In our study, the smear positivity rate of AO stained smears (12.5%) were better than ZN stained smears (11.5%) (Table 2).
Table 2: Comparison of ZN & FL stain. View Table 2
The findings were comparable with those observed by Golia S, et al. [5], Suria J, et al. [6] and Ulukanligil, et al. [7] where the smear positivity rates by ZN were 10.41%, 12.4%, 9.89% and FL were 16.56%, 19.1%, 12.47% respectively.
In the present study, fluorescent microscopy detected additional two positive cases which would have been missed by ZN microscopy alone. However one of them turned out to be negative on culture reflecting either a false positive result or might be an outcome of harsh decontamination of the sample accounting to loss of the viable bacilli [8].
The major strength of our study was comparing the staining methods with culture which is considered as Gold standard for the diagnosis of mycobacterial infection. The sensitivity and specificity of both the staining methods was calculated by comparing the culture results, which adds to the strength to the evidence to the values.
Large number of studies showed that sensitivity of ZN ranged from 32% to 94% and fluorescence microscopy was on average 10% more sensitive than ZN [9].
In the present study, sensitivity of ZN came out to be 91.67% as compared to 95.83% of FL (Table 2). Similar results were obtained by SJ Murray, et al. (93% by FL and 73% by ZN ) [10], K Prashanthi, et al. (69% by FL and 50% by ZN) [11], A Jain, et al. (41% by FL and 32% by ZN) [12], Githui, et al. (80% by FL and 65% by ZN staining) [13] and Ulukanligil, et al. (85.2% by FL and 67.6% by ZN ) [7].
It may be because organisms in FL stain offer better contrast, appearing as brilliant yellow against a dark background. The use of this staining even by some colour blind investigator can be an additional advantage of this technique.
Lower specificity of FL microscopy compared with conventional light microscopy has been reported previously [14]. In the present study, the specificity of FL microscopy (98.86%) was slightly lower than that of ZN microscopy (99.43%) (Table 2). Scanty AFB results on FL microscopy were less likely to be associated with a positive culture result. It is thus more likely that mycobacteria from paucibacillary specimens were killed during decontamination process and failed to grow in culture [8].
False positivity of ZN microscopy and FL fluorescent microscopy is 0.5% (1/200) and 1.0% (2/200) respectively, reported in our study which is not significant. The reasons for false positivity may be specimen from patients on anti-tubercular treatment or processing a bloody sample [15]. The ZN method is also known to give occasional false positive results [16], probably because of the heating step involved in it [17].
In the present study the statistical difference between case detection rates by the two stains was statistically significant (p value < 0.05) (Table 3).
Table 3: Efficacy of ZN & FL in case detection. View Table 3
This was in concordance with studies done in India by Mistry Y, et al. [18], Jain A, et al. [12], Dagar V, et al. [19] as well as abroad by Zailani SB, et al. [20] and Zaib-un-Nisa, et al. [21] where all of them reported a statistically significant difference between case detection by the two staining techniques.
However findings by Subramani P, et al. [22] was in discordance with the present study stating no significant difference between results of the two staining methods.
The sensitivity of smear microscopy is largely determined by the duration of microscopic examination [1]. In high work load settings, the amount of time spent on examining each smear by conventional ZN is low which would probably compromise the sensitivity.
The advantage of fluorescence microscopy is the possibility to scan a smear at 400x magnification rather than at 1000x magnification, allowing theoretical reduction of examination time of the same area to one sixteenth as the surface increases by the square of the diameter. Practically the examination time is reduced to about 10 fold with Fluorescent compared to bright-field microscopy using a 3-fold different [23] magnification (400x vs. 1000x).
In the present study the average time taken to screen per slide by ZN was more (4.32 mins) as compared to that by AO (2.28 mins), reflecting a time saving by 47% (Table 2).
In a study by Tiwari, et al. [24] the mean reading time of Auramine O technique was three times faster than the ZN technique with very good acceptance by the technicians. This was similar to the observation by Marais BJ, et al. [25], where he observed 1.4 minutes to process a slide by FM as compared to 3.6 minutes with conventional ZN microscopy, reflecting a time saving of 61% with FM. Since it is less time consuming hence more slides can be processed in a shorter duration of time.
Despite the fact that conventional fluorescent microscopy has documented higher sensitivity than ZN in huge number of studies and has tremendous potential to reduce laboratory workloads [26], still its incorporation in routine practice has been hampered by various factors including complexity of the microscope, need for a dark room and perceived health risks associated with ultraviolet light exposure [27].
Our findings indicate that FL staining techniques is more effective as far as diagnosis of the disease is concerned, particularly in paucibacillary cases. Taking other factors into account, FL staining has an upper edge in respect to time saving and less observer fatigue. Hence replacement of the age old ZN technique by using FL microscopy may be considered as alternative for diagnosis of tuberculosis after conducting large scale feasibility studies in Indian settings.
Cost issues, however, cannot be ignored which was the short coming of this study.