Lymphadenopathy is common in both adult and paediatric patients in Kenya. It has a broad differential diagnosis, the most common being non-specific reactive lymphoid hyperplasia. Prevalence and morbidity of malignancies in early stages, such as micro lymphoma in reactive lymphadenitis remains poorly investigated.
This study evaluated the histopathologic patterns of lymph node biopsies reported as reactive lymphadenitis at Kenyatta National Hospital between 2013-2019.
Formalin fixed paraffin embedded tissue blocks were used. Consent to conduct the study was obtained from KNH/UoN ERC. Demographic and clinical data were retrieved from the records. Haematoxylin and eosin was performed to confirm the initial diagnosis, followed with immunophenotyping in cases suspicious of lymphomas. Data was analysed using SPSS version 20.
We analysed 86 cases, 64(73%) adults and 22(27%) paediatrics. Age range was 1 year 3 months to 76 years. Males were 49(57%), and females 37(43%). Follicular hyperplasia 63(73%), sinusoidal 16(19%) and PTGC 1(1%) were the histopathological patterns reported. No diffuse or mixed patterns, and no micro lymphomas were found. However, immunophenotyping confirmed 6(7%) cases to be DLBCL (1 case), high grade round blue cell tumour of childhood (1 case), and 4(3SLL and 1MCL) cases.
Diagnosis of reactive lymphadenitis is a major challenge, especially in settings with limited utilization of immunophenotyping and molecular analyses. It has a broad differential diagnosis, the most common being nonspecific reactive lymphoid hyperplasia, which often disguises malignancy. In this study, follicular hyperplasia was the more common sub-classification.
Sub-classifications of reactive lymphadenitis may help in diagnosis of underlying pathologic conditions.
Lymphoid hyperplasia, Reactive lymphadenitis, Micro lymphomas, Immunophenotyping, Sub-classification
Non-specific reactive hyperplasia, a benign reversible enlargement of the lymph node, is the leading cause of lymphadenopathy. It has a higher incidence in the cervical, compared to the inguinal region [1-3]. Lymphadenopathy has been reported as a common clinical finding, with a broad differential diagnosis, including lymphoma [4,5].
When lymphoma features as differential diagnosis in lymphadenopathy, there has been only paucity of reported data on the prevalence of micro lymphoma, particularly when ancillary testing is not utilized alongside morphological evaluation [6-8]. In such instances, lymphadenopathies have often been diagnosed as reactive lymphadenitis, but later found to be of specific pathologic processes [9,10]. A variety of unusual reactive conditions have also been reported in lymph nodes in the pediatric age group, which are of unknown cause [11]. However, the prevalence and morbidity of malignancies in early stages, e.g. micro lymphoma, in diagnoses of reactive lymphadenitis have been poorly investigated, and limited information is available on studies from the developing countries [12,13].
Distinct diagnosis of lymphadenitis and malignant lymphoma requires good knowledge of the basic forms of the disease, as well as in depth knowledge of the structure of the individual lymph node compartments [14-16]. There are defined forms of lymphadenitis where the differential diagnosis to certain lymphoma entities are known. Other reactive structural alterations show indistinct limits, and are only possible to ascertain the correct diagnosis by clinical, morphological, immunophenotype and molecular features according to the WHO recommendation [17,18].
In our setting, apart from the problem of late presentation of the disease at the time of referral of the patients, there is sub optimal utilization of ancillary testing, due in part to limited infrastructural support and skills [19-21]. It is therefore likely that cases reported as lymphadenitis could have lymphomas, but are largely missed or under diagnosed. In order to improve on the diagnosis and institute definitive management intervention for lymph proliferative disorders, it is only necessary that the attending physicians have thorough knowledge of the demographic pattern of reactive lymphadenitis [22]. There has also been a change in the epidemiology of lymph node diseases over the years. Lymphomas remain a leading cause of cancer mortality, especially with the advent of HIV/AIDS, underscoring the need for more studies in our environment [23].
It is on the basis of this background information that the present study sought to give an insight on the sub-classification of the morphologic spectrum of reactive lymphadenitis seen in our facility, and to determine the prevalence of micro lymphomas in the studied cases.
Approval to conduct the study was obtained from KNH/UoN ERC. There was no contact with the patients, and request for exemption for informed signed consent was included in the application for ethical approval. All procedures performed in the current study were approved by KNH/UoN research ethics committee (reference number P115/02/2020, and dated 08th May, 2020) in accordance with the 1964 Helsinki declaration and its later amendments. Formalin fixed paraffin embedded (FFPE) tissue blocks of lymphadenopathy, previously reported only as reactive lymphadenitis on histopathology, in the department of human pathology at KNH were used for the study.
Ethical approval to conduct the study was obtained from KNH-ERC. Demographic and clinical data were retrieved from the records. These included the age of the patient, anatomical site, duration of lymphadenopathy, preliminary and final diagnoses. Previous histopathological diagnoses were obtained from histology register.
Formalin fixed paraffin embedded tissue blocks of cases reported as reactive lymphadenitis were retrieved from the archives once the data was scrutinized and verified. Morphological evaluation was done to confirm the initial reported diagnosis of non-specific reactive hyperplasia. Briefly, 4μm sections from the tissue blocks were stained with hematoxylin and eosin to confirm the previous diagnoses and establish the tumor grades according to the WHO guidelines.
To further characterize the tumors, immunophenotyping was done in cases that were equivocal on morphology, with features suspicious of lymphomas. In brief, immunohistochemistry was done on 4μm sections cut from the FFPE tissue blocks and float mounted on positively charged slides as previously described [24]. The slides were incubated in a 56-60 °C oven for 15 minutes and subsequently deparaffinized in xylene bath, with two changes of xylene for 5 min each. The slides were rehydrated in two changes of fresh absolute ethanol for 3 min each, followed by 90% and 80% alcohol, respectively for 3 mins each. Antigen retrieval was done using protease treatment. All the slides were incubated with the respective primary antibodies for 1 hour at room temperature, rinsed with tris buffered saline tween-20 (TBST) and then incubated with secondary antibody (ABC kit, DAKO Cytomation) for another 30 minutes. The sections were rinsed and streptavidin peroxidase was applied, followed by incubation for another 30 minutes. The sections were rinsed with TBST and then stained with DAB for 7 minutes. The sections were then rinsed again, counter-stained with Meyers haematoxylin and mounted. The clones and source of the primary antibodies used are indicated in Table 1. All the dilutions were made at 1:50. In each case, complete cell counts were evaluated in five different fields at 40X and positive cells were expressed as mean percentages against the total cells in the fields. The slides were reviewed by two independent experienced pathologists from which 98% concordance was achieved. In case of discordance, the slides were simultaneously reviewed by the two Pathologists to achieve a consensus.
Table 1: Panel of antibodies used for immunohistochemistry. View Table 1
Data was processed using Epi-info, and analyzed using SPSS version 20. Frequencies were generated and represented in figures and tables. Measures of central tendency were generated for continuous variables such as age. A Shapiro-Wilk normality test was performed on all continuous variables to determine the distribution pattern of the data. The statistical tests were performed and set at 95% confidence interval.
A total of 86 cases were analyzed, and included 64(73%) adults and 22(27%) pediatrics. Age range was 1 year 3 months to 76 years, with a median age of 30 years with an interquartile range of 16-47 years. Males were 49(57%), and females 37(43%) (Figure 1).
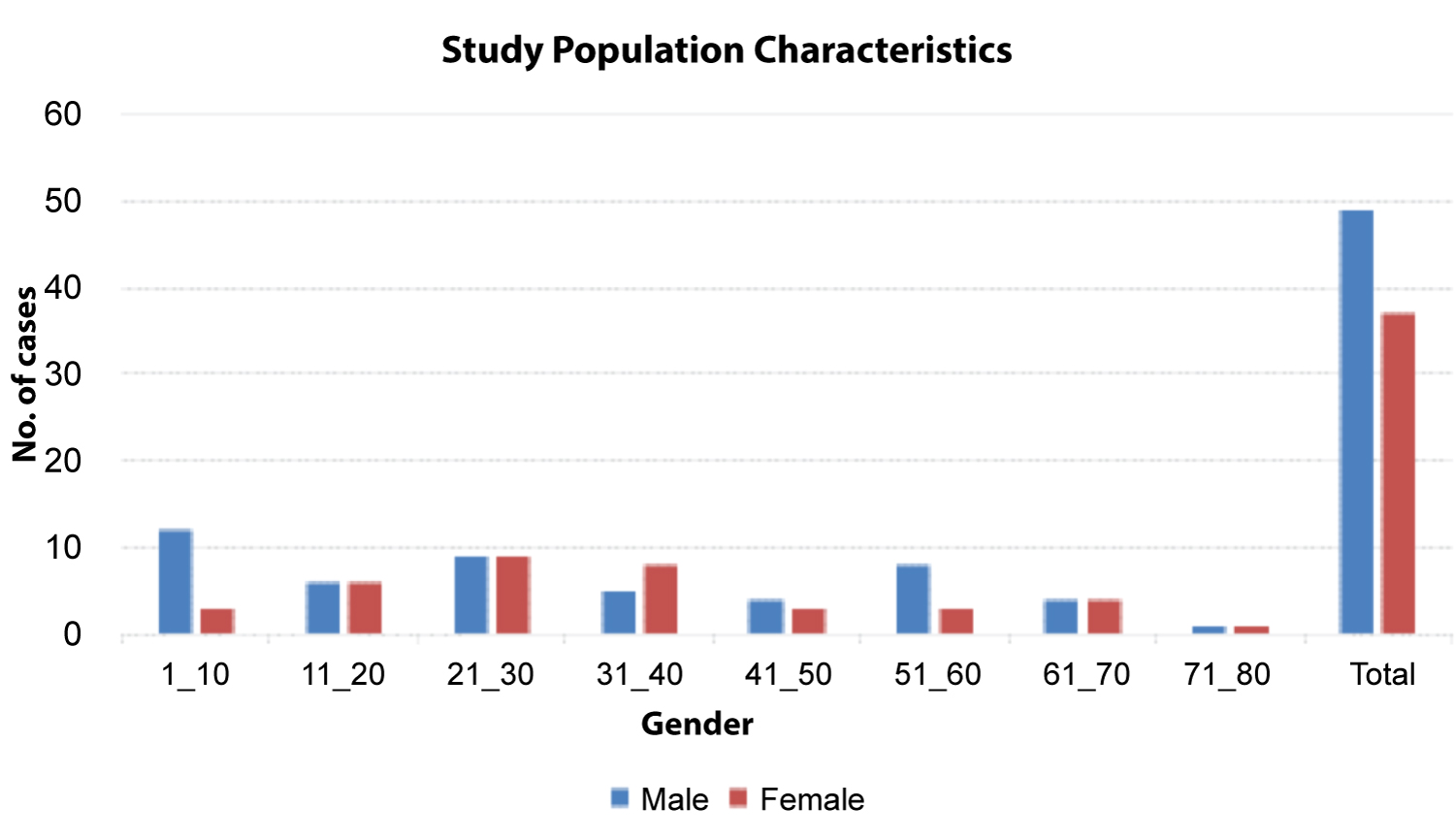 Figure 1: Study population characteristics.
View Figure 1
Figure 1: Study population characteristics.
View Figure 1
In terms of distribution of the cases per site, there was a higher frequency of clinical presentation of reactive lymphadenitis in the cervical region of the neck (52%), compared to the inguinal region (9%) (Table 2).
Table 2: Distribution of reactive lymphadenitis based on site. View Table 2
The histopathological patterns recognized included follicular hyperplasia 63(73%), sinusoidal 16(19%) and progressive transformation germinal center (PTGC) 1(1%) (Table 3, Figure 2, Figure 3 and Figure 4). More females than males presented with the follicular pattern of reactive lymphadenitis (78% vs. 65%), p = 0.186. Only males presented with the sinusoidal pattern. No diffuse or mixed patterns, and no micro lymphomas were found. However, immunophenotyping confirmed 6(7%) cases to be DLBCL (1 case), high grade round blue cell tumor of childhood (1 case), and 4(3SLL and 1MCL) cases (Figure 5, Figure 6, Figure 7 and Figure 8).
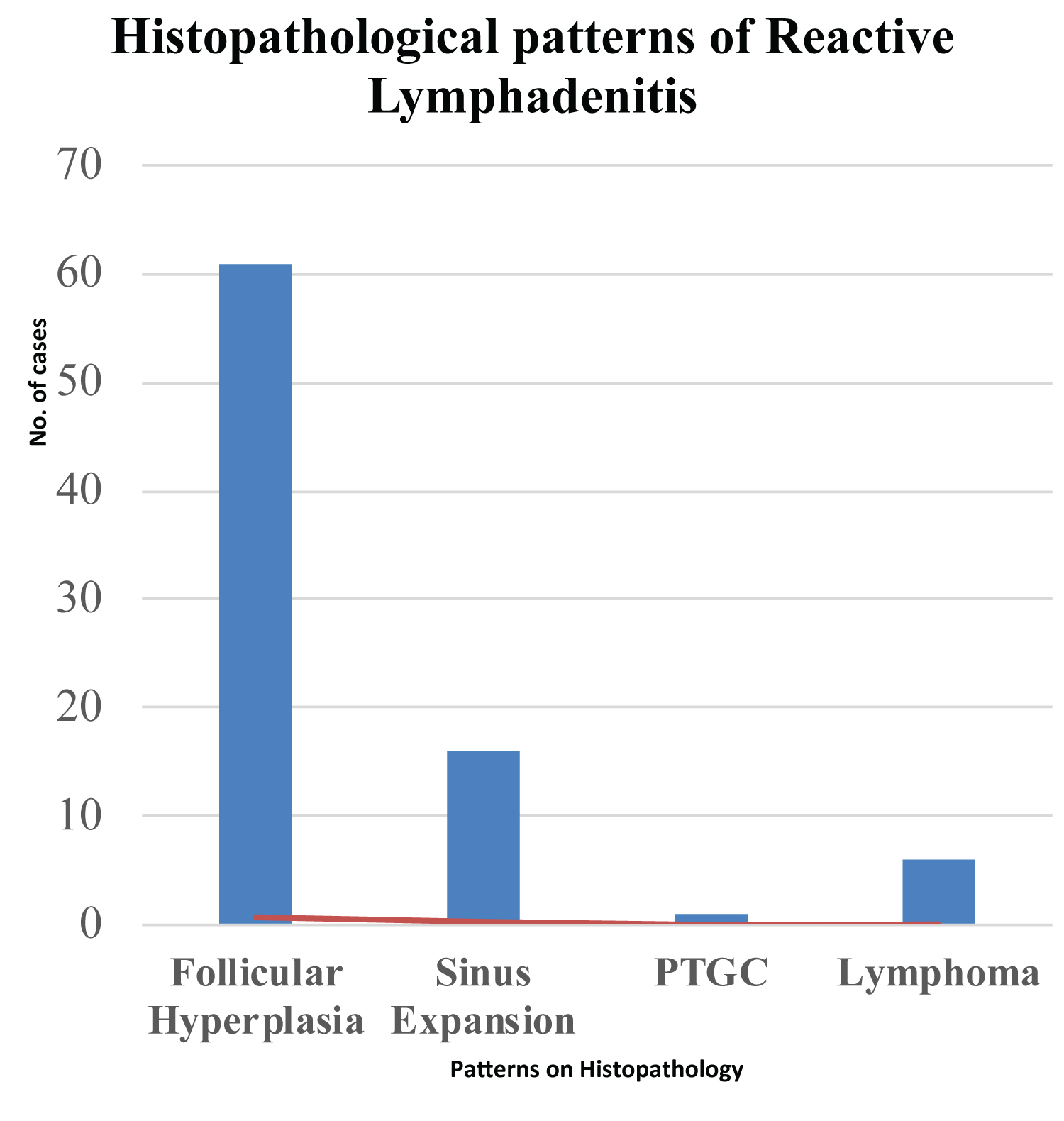 Figure 2: Histopathological patterns of reactive lymphadenitis in the studied cases.
Figure 2: Histopathological patterns of reactive lymphadenitis in the studied cases.
PTGC: Progressive Transformation Germinal Center.
View Figure 2
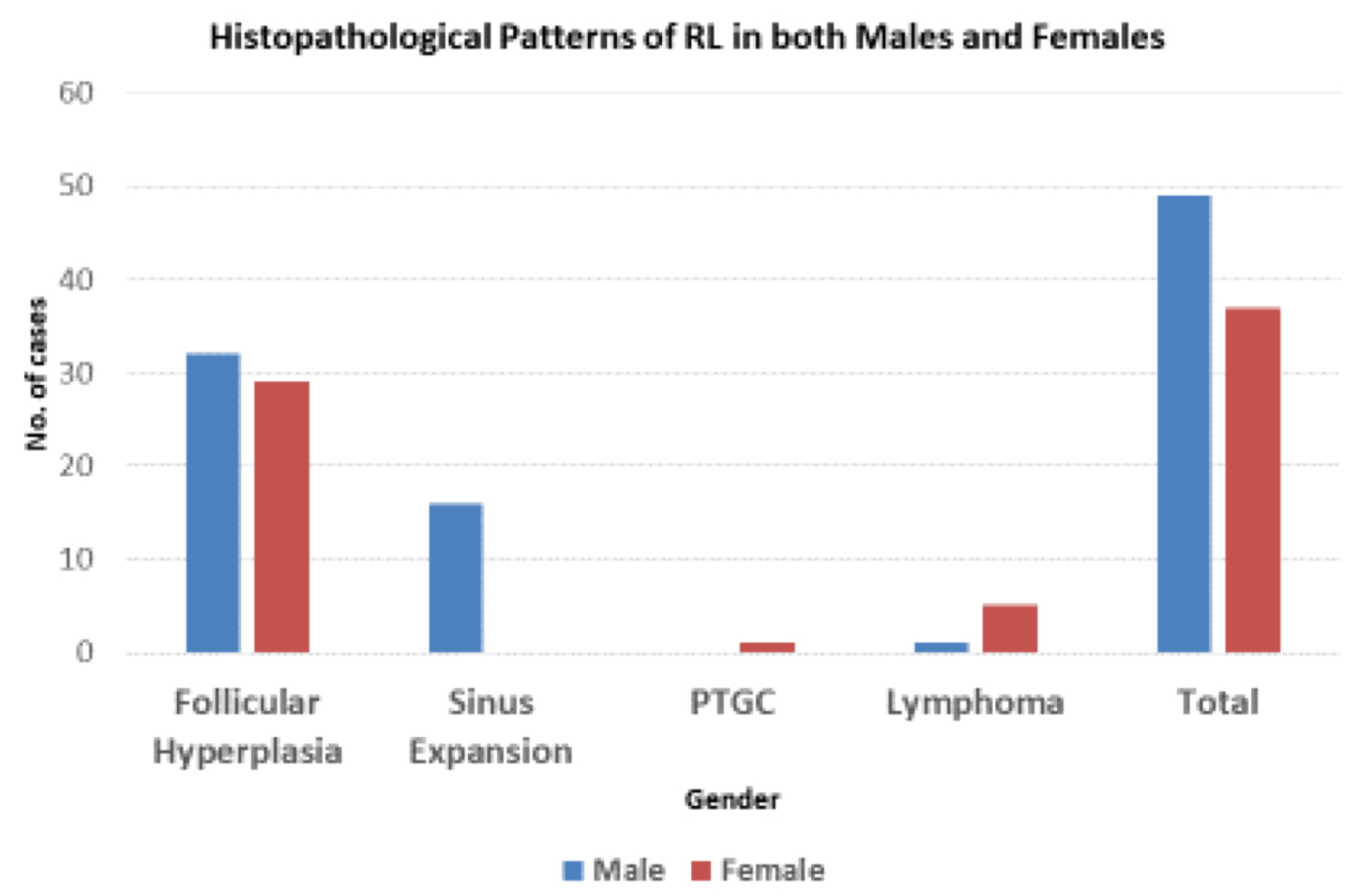 Figure 3: Histopathological patterns of reactive lymphadenitis in both males and females.
View Figure 3
Figure 3: Histopathological patterns of reactive lymphadenitis in both males and females.
View Figure 3
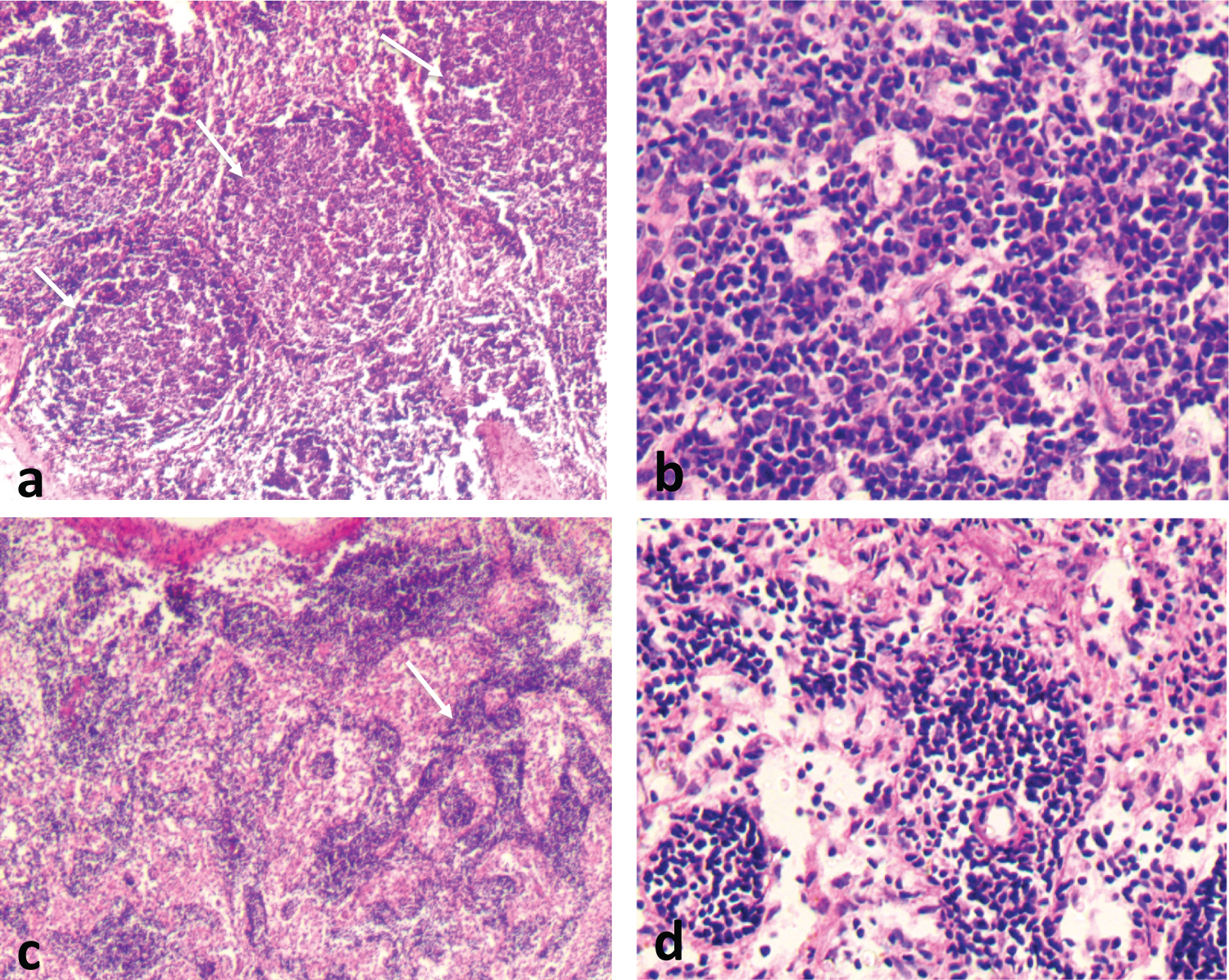 Figure 4: Reactive Lymphadenitis, Follicular Pattern. Reactive lymphadenitis is characterized by proliferation of cellular components of the lymph node and preservation of the general architecture as shown (a). The follicular pattern is preserved (arrows), with no capsular infiltration. Follicles vary in size and shape and are composed of centrocytes and centroblasts (b). Tingible body macrophages are common. Sinus expansion to the capsule (arrows), (c) and (d) are seen.
View Figure 4
Figure 4: Reactive Lymphadenitis, Follicular Pattern. Reactive lymphadenitis is characterized by proliferation of cellular components of the lymph node and preservation of the general architecture as shown (a). The follicular pattern is preserved (arrows), with no capsular infiltration. Follicles vary in size and shape and are composed of centrocytes and centroblasts (b). Tingible body macrophages are common. Sinus expansion to the capsule (arrows), (c) and (d) are seen.
View Figure 4
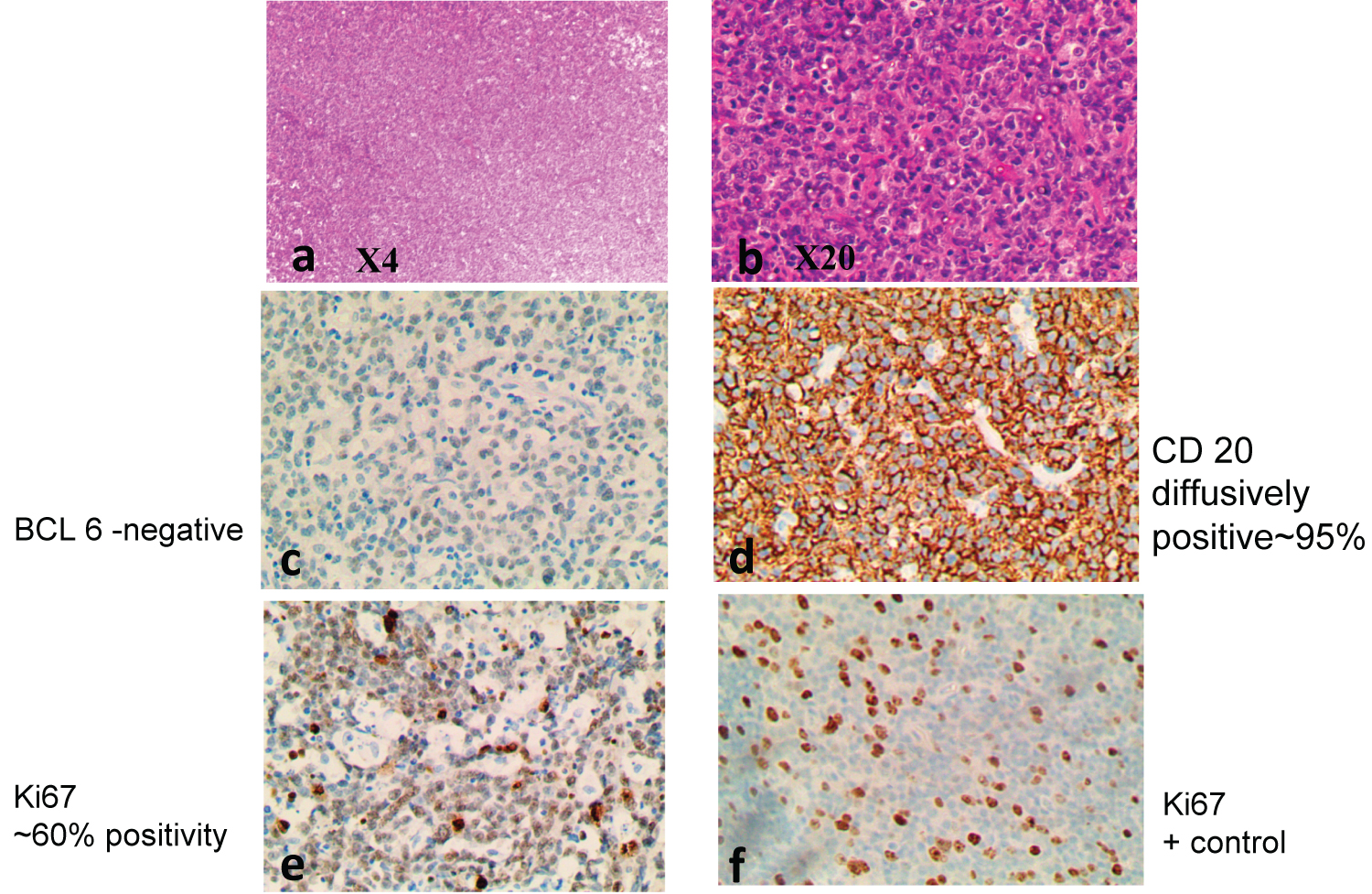 Figure 5: DLBCL exhibiting diffuse growth pattern, (a), with large cells (b). IHC confirms DLBCL of the Activated B cell type, with CD 10 positive (not shown), MUM1 negative (not shown), BCL 6 negative (c), CD 20 positive (d), Ki67 ~ 80% positivity (e).
View Figure 5
Figure 5: DLBCL exhibiting diffuse growth pattern, (a), with large cells (b). IHC confirms DLBCL of the Activated B cell type, with CD 10 positive (not shown), MUM1 negative (not shown), BCL 6 negative (c), CD 20 positive (d), Ki67 ~ 80% positivity (e).
View Figure 5
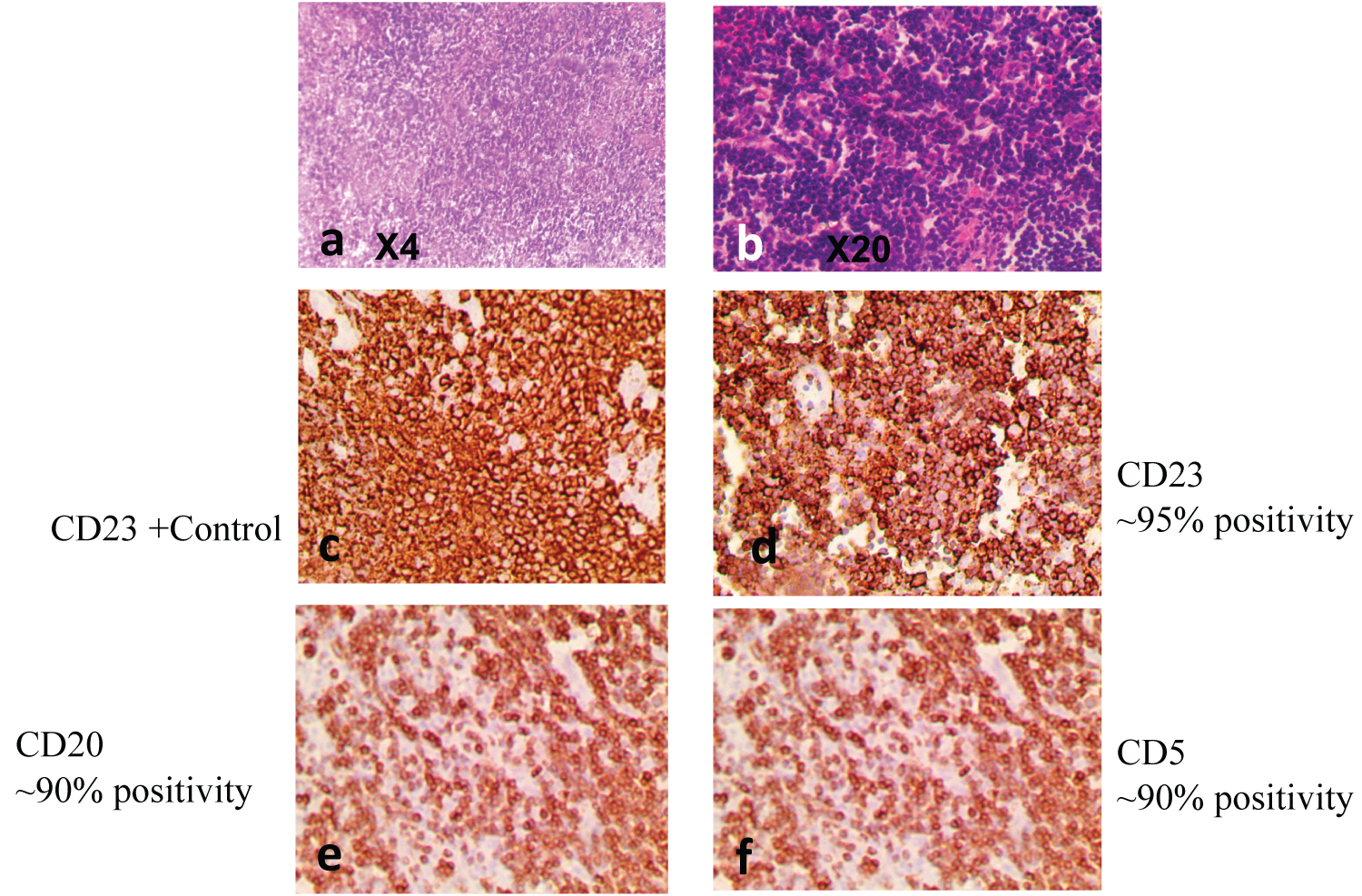 Figure 6: Small Lymphocytic Lymphoma, characterized by diffuse effacement of parenchyma by small mature lymphocytes (a and b). IHC confirmed CD 23-positivitve (d), CD20-positive (e), and CD5-positive (f).
View Figure 6
Figure 6: Small Lymphocytic Lymphoma, characterized by diffuse effacement of parenchyma by small mature lymphocytes (a and b). IHC confirmed CD 23-positivitve (d), CD20-positive (e), and CD5-positive (f).
View Figure 6
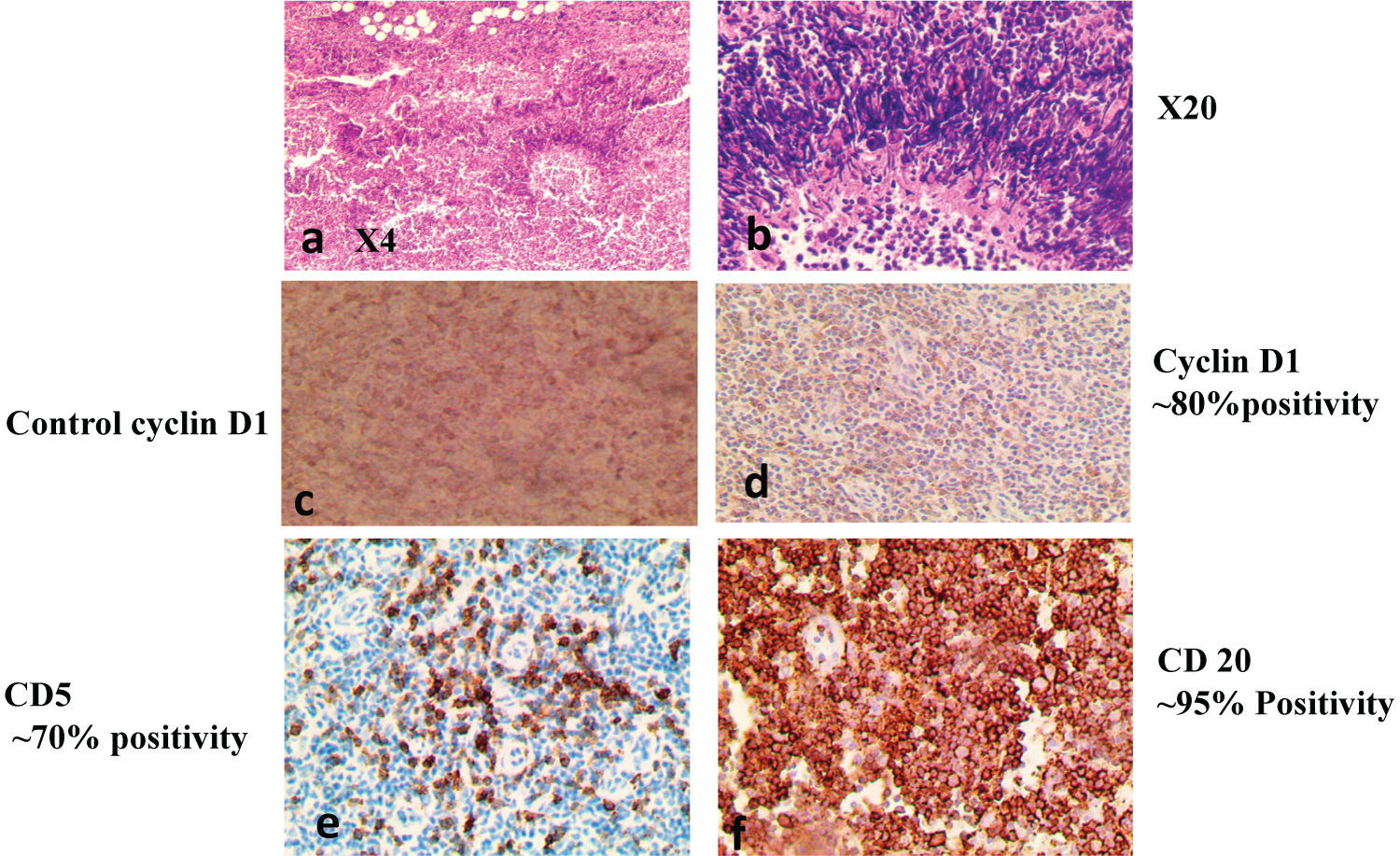 Figure 7: Mantle Cell Lymphoma on H/E at (X4) and at (X20). IHC positive for MCL-Cyclin D1 (d), CD5 (e), CD10 (not shown), CD20 (f).
View Figure 7
Figure 7: Mantle Cell Lymphoma on H/E at (X4) and at (X20). IHC positive for MCL-Cyclin D1 (d), CD5 (e), CD10 (not shown), CD20 (f).
View Figure 7
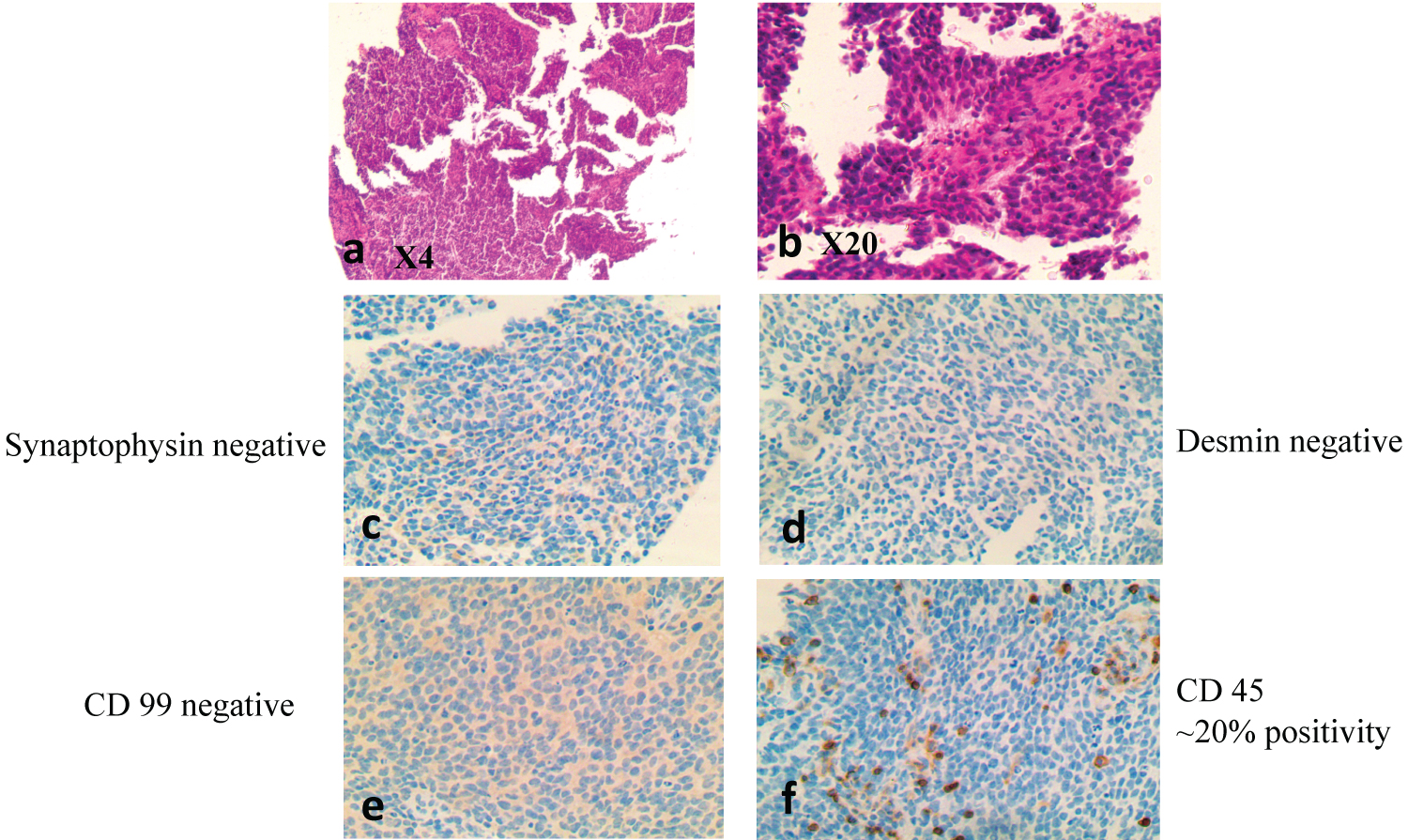 Figure 8: Round blue cell tumor of childhood, characterized by primitive high grade round blue cells on H/E (X4) and (X20) (a and b), respectively. IHC showed Synaptophysin negative (c), Desmin-negative (d), CD 99-negative (e) and CD45-negative (f).
View Figure 8
Figure 8: Round blue cell tumor of childhood, characterized by primitive high grade round blue cells on H/E (X4) and (X20) (a and b), respectively. IHC showed Synaptophysin negative (c), Desmin-negative (d), CD 99-negative (e) and CD45-negative (f).
View Figure 8
Table 3: Histopathological patterns based on gender. View Table 3
This study reviewed the morphologic spectrum of cases reported as reactive lymphadenitis at KNH. A total of 86 cases were reviewed in the seven-year period from 2013-2019. The study population comprised of 49(57%) males and 37(43%) females. Of the 86 cases analyzed, 64(73%) were adults while 22(27%) cases were of the pediatrics. This age distribution is similar to other previous studies in Africa [2,3].
There was a higher frequency of clinical presentation of reactive lymphadenitis in the cervical region of the neck 45(52%), and was consistent with studies done in South Sudan which reported 69.4% cervical nodal presentation in their study cases [25]. Similar finding was also reported in a study done in Nigeria, with 46% of their study population showing cervical node involvement [20]. This may be related to its location near a common primary site of infection and the fact that these nodes drain the upper aero-digestive tract through which foreign antigens enter the body via ingestion or inhalation.
In the present study, the most frequent pattern of reactive lymphadenitis was follicular hyperplasia 63(73%). This finding was similar to a study done in Saudi Arabia that showed follicular hyperplasia as the predominant pattern of reactive lymphadenitis at 60% [26]. Other studies in South Africa and Nigeria reported similar findings, at 68% and 66%, respectively [8,20]. A wide variety of conditions such as infection, drugs, chemicals, environmental pollutants, and even malignancy are often associated with lymphadenitis. However, infections and autoimmune conditions associated with this pattern are common in our set up and could explain these findings.
Reactive sinus hyperplasia was found to be the second most common pattern of reactive lymphadenitis, similar to studies done in SA and Nigeria [2,8,27]. This is seen in relation with monocytoid B-cell hyperplasia, hemophagocytic syndromes, Whipple's disease, or lymph nodes draining sites of prostheses or malignancy.
PTGC was found to be the least common histopathological pattern of reactive lymphadenitis, similar to studies done in the US and Nigeria, where it was found to be at 2% and 1% respectively [19,28].
There was no micro lymphoma found in cases reported as reactive lymphadenitis at KNH. This can be attributed to late health seeking behavior in our set-up, for which patients present with advanced disease stage of lymphomas. In our study, lymphoma was found in 7% of cases reported as reactive lymphadenitis. Studies in the US and SA found lymphoma in 17% of cases reported as reactive lymphadenitis while in Nigeria it was found at 6% [3,8,9]. This can be attributed to advanced healthcare systems and early health seeking behavior patterns in the high-income countries, compared to that in our set-up and also to earlier diagnosis of malignancies before onset of nodal metastases.
The need for sub-classifications in the diagnosis of reactive lymphadenitis cannot be overemphasized, as they may reveal underlying pathologic conditions and improved diagnosis for better management and disease outcome in patients seen in Kenyatta National Hospital.
The authors declare no conflict of interest in this study.
Data curation: CCC, JN, MG; Formal analysis: CCC, JN, MG; Investigation: CCC, JN, JRN, MG, SL, LL; Methodology: CCC, JN, JRN, MG; Project administration: CCC, JN, JRN, LL; Resources: CCC, JN, JRN, MG, SL, LL; Software: CCC, JN; Supervision: JN, JRN; Validation: CCC, JN, JRN; Visualization: CCC, JN, JRN, LL; Writing - original draft: JN, CCC; Writing - review & editing: JN, CCC, LL.
The authors would like to thank Prof. Lorenzo Leoncini from University of Siena, Italy for the review of the pathology slides.