Fibromatosis is a cellular spindle cell lesion that may mimic dilemma of other spindle cell lesions, thus many tools should be available for differentiation including immunohistochemical markers. Nuclear beta catenin is a well known diagnostic marker for fibromatosis but with limited specificity and frequent background stain. Calretinin is an intracellular calcium binding protein that is found to be expressed in many cell types.
Evaluate the expression of calretinin in abdominal wall and intra-abdominal types fibromatosis as well as other mimic spindle cell lesions in a trial to improve the diagnostic accuracy of deep fibromatosis when other spindle cell lesions are suspected.
The study included 20 desmoid cases (abdominal wall fibromatosis) along with 7 cases of intra-abdominal mesenteric fibromatosis and other spindle cell lesions including 11 MPNST (malignant peripheral nerve sheath tumor) cases, 15 cases LMS (leiomyosarcoma), 5 cases synovial sarcoma, 9 cases SFT (solitary fibrous tumor), 19 cases GIST (gastrointestinal stromal tumor), 12 cases Schwannoma and 13 cases neurofibroma which were retracted from the pathology files at Ain Shams University Hospitals with revision of all their available clinical data and available stained sections and then subjected to calretinin immunostain.
Calretinin immunohistochemical expression was positive in 85.1% of deep fibromatosis cases including cases of desmoid tumor (18/20) and mesenteric fibromatosis (5/7) (48.1% patchy and 37% diffuse), 33.3% of LMS were presented by diffuse staining, 83.3% of schwannoma were presented by patchy staining. While all cases (100%) of MPNST, synovial sarcoma, SFT and GIST were negative for calretinin. Sensitivity was 85.2% and specificity 80.9% with positive predictive value 59% and negative predictive value 94%.
Calretinin can be used to raise the diagnostic accuracy of fibromatosis versus other spindle cell lesions especially when MPNST, synovial sarcoma, SFT, and GIST are considered in the differential diagnosis. Calretinin is also a good diagnostic marker for schwannoma.
Desmoid tumour, Deep fibromatosis, Immunohistochemistry, Calretinin, Spindle cell lesions
MPNST: Malignant Peripheral Nerve Sheath Tumor; LMS: Leiomyosarcoma; GIST: Gastrointestinal Stromal Tumor; SFT: Solitary Fibrous Tumor
Deep fibromatosis represents clonal myofibroblastic proliferations that are prone to aggressive local recurrences but do not metastasize. It can develop as intra-abdominal, abdominal wall or extra-abdominal with all share the most of clinical, morphologic, immunohistochemical, and molecular genetic features [1]. The diagnosis of abdominal wall desmoid tumor is usually straightforward by use of clinical and histopathological examination of hematoxylin and eosin (H&E) stained sections that are enough to reach a definite final diagnosis. On the other hand, the intra-abdominal and the extra-abdominal lesions sometimes show dilemma of the differential diagnoses and mostly immunohistochemical markers are added to reach a definite final diagnosis [2].
Intra-abdominal fibromatosis, that developed from mesentery mainly, but also may develop from omentum and retroperitoneum, is the most common primary mesenteric tumor with most of the cases occurred sporadic but some cases are associated with Gardner syndrome. It is challenging entity with many other intra-abdominal and retroperitoneal spindle cell lesions including sclerosing mesenteritis, inflammatory myofibroblastic tumor, solitary fibrous tumor, desmoplastic peritoneal mesothelioma, retroperitoneal spindle cell lesions especially well differentiated leiomyosarcoma, neuronal tumors, and idiopathic retroperitoneal fibrosis (Ormond's disease) and with the most confusing entity is mesenteric GIST (gastrointestinal stromal tumor) especially if fibromatosis encroaching on the intestinal wall [3]. Although, there are some criteria on H&E stained sections can help in differentiation, definite diagnosis mostly need application of immunostains. Many immunohistochemical markers were tried and become employed to reach a definite final diagnosis for each lesion as DOG1 marker for GIST [4].
Extra-abdominal fibromatosis may also resemble low grade malignant spindle cell lesion on one extreme and the reactive process on the other with the differential diagnosis including dilemma of lesions including differentiated fibrosarcoma, low grade fibromyxoid sarcoma, low grade myofibroblastic sarcoma, well differentiated leiomyosarcoma, myofibroma/myofibromatosis on one hand and include nodular fasciitis and scar on the other hand [5]. Moreover, in breast lesions, pathologist should exclude low grade metaplastic carcinoma and malignant phyllodes tumor before considering the diagnosis of fibromatosis [6,7].
Strong nuclear staining to beta catenin is commonly used for further confirmation of such diagnosis, but with poor specificity as one of the studies was found that beta catenin was expressed in 30% low-grade myofibroblastic sarcomas, in 22% of solitary fibrous tumours, 20% of in infantile fibrosarcoma, in 6% of desmoplastic fibroblastomas and in 5% of gastrointestinal stromal tumor [8]. Also, it is difficult for interpretation because of background/nonspecific staining [2,9].
Most sporadic desmoid tumors are associated with mutations of the β-catenin gene (CTNNB1) that can be used as a specific diagnostic tool for the diagnosis of desmoid tumors, but molecular tests are still not widely used in the countries with limited resources as it is much more expensive than immunohistochemical studies and very limited if any molecular laboratories are available in these countries [10].
Calretinin is a 29 KD calcium-binding protein and a member of the family to which S-100 also belongs. Its function is thought to be to buffer intracellular calcium. It is expressed by different types of cells including mesothelial, epithelial and stromal cells. It is the most common used marker to confirm mesothelioma [11], and it is widely used to confirm other neoplastic lesions as sex cord stromal tumors, ameloblastoma and cardiac myxoma [7].
In the present study, we aimed to evaluate the expression of calretinin in deep fibromatosis cases (20 desmoid/abdominal wall) and 7 intra-abdominal (mesenteric) that were diagnosed on clinical and pathological assessment of the lesions as a trial to improve the diagnostic accuracy of fibromatosis lesion when other spindle cell lesions are suspected.
The present study was conducted on 27 deep fibromatosis cases (20 desmoid/abdominal wall and 7 mesenteric/intra-abdominal) that were collected from the pathology files of the Pathology Department at Ain Shams University Hospitals during the period from January 2017 to December 2018. All cases were females with age ranging from 23 to 35 years and were presented by anterior abdominal wall mass for abdominal wall desmoids and mostly by intestinal obstruction for intra-abdominal mesenteric lesions. Cases were diagnosed clinically and histopathologically including positive stain to nuclear beta catenin. Out of the 27 studied cases there were 5 cases presented as core biopsy with all the remaining cases (22) were excision biopsy. Other spindle cell lesions which were confirmed by histopathological +/- immunohistochemistry for S100, SOX10, CD56, CD57, Protein gene product 9.5, SMA, H caldesmon, CD34, C-kit, DOG 1, EMA, Bcl2, CD99, TLE 1 and CK were included in study as follows (MPNST: 11 cases, Schwannoma: 12 cases, neurofibroma: 13cases, LMS: 15 cases, GIST: 19 cases, synovial sarcoma: 5 cases and SFT: 9 cases).
The stained sections were revised by the two authors to verify the histopathological diagnosis and to select the best representative sections for immunohistochemical study.
The material used in this study included only paraffin blocks as it was a retrospective and the patients were unknown to the authors, and patients identity were anonymous in the study design. Hence, no consent from the patient was required.
One paraffin block that best represented the lesion was selected for all cases. 5 microns thick tissue sections were cut from the paraffin blocks on positive charge slides. Deparaffinization and rehydration were performed by treating the tissue sections in a microwave oven for 5 min at 700 W and then in Diva Decloaker antigen retrieval solution that was diluted in distilled water in a ratio of 1:10. Two drops of hydrogen peroxide block were then added for 15 min. Immunohistochemical staining was performed at room temperature using an immunostainer (Shandon Sequenza Immunostainer, Thermo Scientific, Astmoor, Runcorn Cheshire, UK). A volume of primary 100 micron of primary antibody was applied to each section, followed by overnight incubation. Calretinin was a Rabbit monoclonal antibody (6 ml ready to use clone SP13 Cat.No. GTX79427, Gene Tex, USA). Secondary antibody (Ultravision large volume detection system kit, antipolyvalent, horseradish peroxidase, ready to use, Cat. #: TP-060-HL; Lab Vision Westinghouse Dr, Fermont, California, USA) was added to each section for 30 min, followed by addition of DAB and substrate (Cat.#: TA-060-HDX; Lab. Vision) and incubation for 5 to 10 min. Rinsing with distilled water was performed after each step. Sections were counter stained with Harris Hematoxylin and then mounted using canada balsam. One section from mesothelioma was included in each run as positive control for calretinin whereas the primary antibody was replaced by non-immune IgG for the negative control prepared section.
We considered the intensity and the percentage of positive cells in the interpretation of calretinin immunostain. Thus, staining intensity was graded into mild, moderate or strong stain and the average percentage of positive cells were estimated, if less than or about 50% of tumor cells were positive, patchy/focal stain was considered and if more than 50% of the cells were positive, diffuse stain was considered. If less than 5% of the tumor cells were positive, negative stain was considered. The site of the stain was either nuclear only or nuclear and cytoplasmic.
Statistical analysis was performed using statistical package for the social science (IBM SPSS Statistics for Windows, version 20.0; IBM, New York, New York, USA). Qualitative variables were expressed as frequency and percent. Fisher’s exact test and McNemar’s test were used as appropriate. The predictive capacity of calretinin immunostaining was determined using sensitivity, specificity, positive predictive value, and negative predictive value (NPV) calculations.
This study included 111 cases with an age range from 20 to 76-years-old (mean: 37.77 ± 10.19). The cases comprised 69 females (62.2%) and 42 males (37.8%) with the anterior abdominal wall was the most common location in 20 cases (18%). 94 cases were obtained by excision biopsy 94 (84.7%), while 17 cases were obtained by tru cut biopsy (15.3%) (Table 1).
Table 1: Gross description of studied specimens. View Table 1
Calretinin immunohistochemical expression was positive 85.1% of cases of deep fibromatosis cases (in 18 out of 20 abdominal wall cases and in 5 out of 7 mesenteric cases) (48.1% patchy and 37% diffuse) (with 7.4% of the cases showed nuclear stain only, 77.8% were showing both cytoplasmic and nuclear staining) (Figure 1A, Figure 1B, Figure 1C and Figure 1D), 83.3% of schwannoma were presented by patchy nuclear staining (Figure 1E) and 33.3% of LMS were presented by diffuse cytoplasmic along with patchy nuclear staining (Figure 1F), While all cases (100%) of MPNST, neurofibromas, synovial sarcoma, SFT and GIST were negative for Calretinin. There was a statistically significant difference between calretinin expression in deep fibromatosis versus other spindle cell tumors as regards staining intensity, extent and cellular localization (Table 2, Table 3 and Table 4).
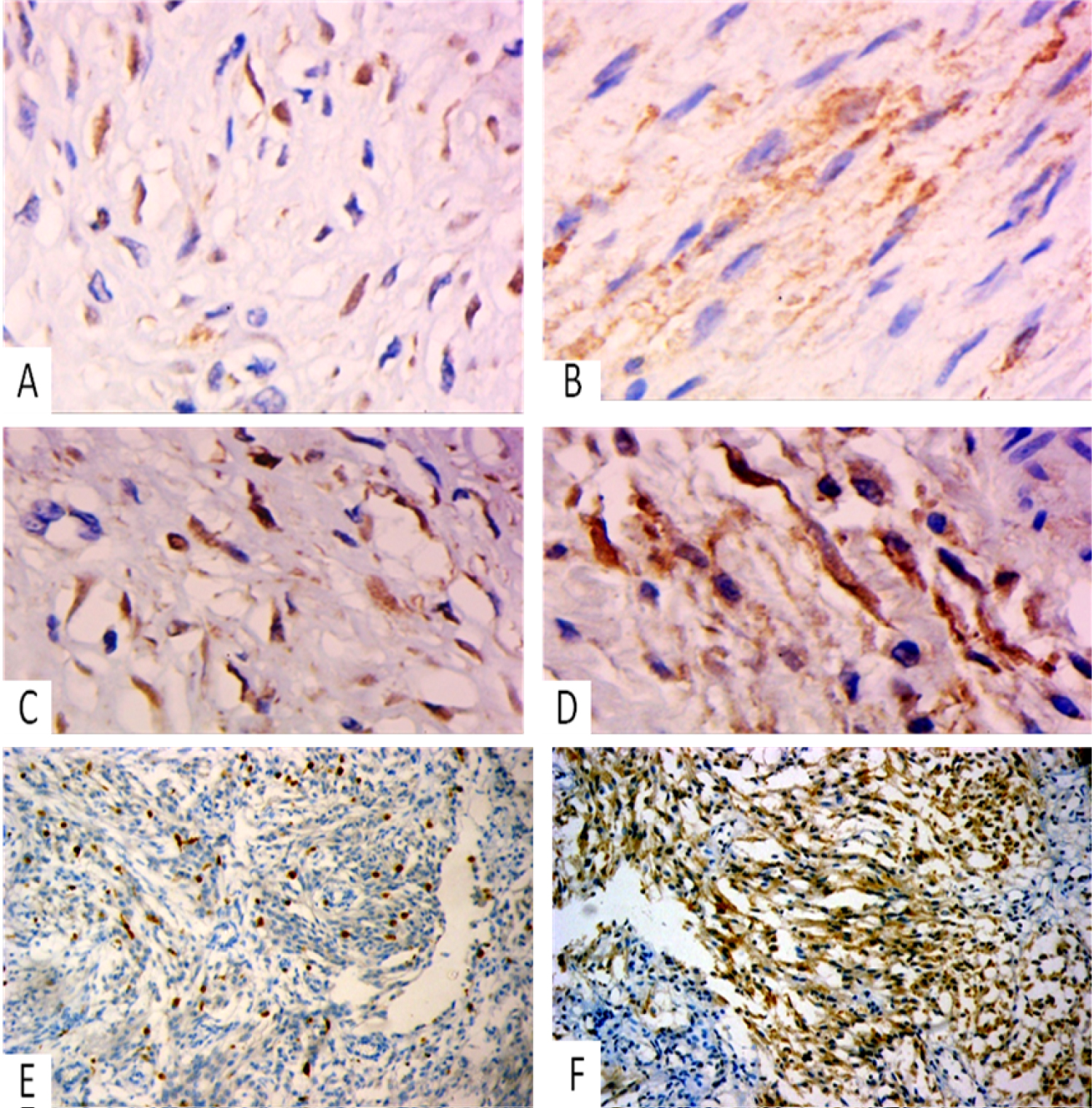 Figure 1: (A-D) Fibromatosis shows positive patchy moderate to strong calretinin nuclear and cytoplasmic stain x400; (E) Schwannoma shows positive nuclear calretinin stain, x200; (F) Leiomyosarcoma shows diffuse positive cytoplasmic and patchy nuclear calretinin stain, x200.
View Figure 1
Figure 1: (A-D) Fibromatosis shows positive patchy moderate to strong calretinin nuclear and cytoplasmic stain x400; (E) Schwannoma shows positive nuclear calretinin stain, x200; (F) Leiomyosarcoma shows diffuse positive cytoplasmic and patchy nuclear calretinin stain, x200.
View Figure 1
Table 2: Difference between deep fibromatosis and other types of spindle cell tumors regarding calretinin expression. View Table 2
Table 3: Difference between deep fibromatosis and LMS regarding calretinin expression. View Table 3
Table 4: The difference between deep fibromatosis and Schwannoma regarding calretinin expression. View Table 4
Regarding the diagnostic accuracy of calretinin in differentiating deep fibromatosis from other spindle cell tumors; sensitivity was 85.2% and specificity 80.9% with a positive predictive value 59% and negative predictive value 94%.
Although immunostaining to nuclear beta catenin is used regularly to assess difficult cases of deep fibromatosis, it showed low specificity. Thus, it is recommended to have a panel of makers to assess deep fibromatosis especially when dilemma of the differential diagnosis considered. Deep fibromatosis could be abdominal wall, intra-abdominal and extra-abdominal, with all are indistinguishable grossly and microscopically [6]. Reitamo, et al. [12] reported that abdominal wall fibromatosis has outnumbered (49%) the extra-abdominal (43%) and mesenteric fibromatosis (8%).
Arva NC, et al. [13] and Ahmed, et al. [14] reported large list of differential diagnosis for fibromatosis including fibrosarcoma, reactive fibrosis as nodular fasciitis , solitary (benign or malignant) fibrous tumor, leiomyoma, and GIST (spindle cell type) in mesenteric location.
Barak, et al. [15] added many entities to the differential diagnosis list including low grade myofibroblastic sarcoma and fibromyxoid sarcoma, sclerosing mesenteritis/idiopathic retroperitoneal fibrosis and fibrous scar. They used nuclear beta catenin in collaboration with other markers and histopathological parameters to differentiate these lesions.
Sophie Le Guellec, et al. [10] reported that positive nuclear staining for beta catenin in fibromatosis is a good diagnostic tool after they studied 260 desmoid tumors and 191 potential morphologic mimics. Montgomery and Folpe, [16], Ng, et al. [17] and Amary, et al. [2] reported that strong nuclear staining of beta catenin is nearly 100% sensitive for all deep fibromatosis but lack specificity. Army, et al. [2], found that 72% of the lesions mimicking fibromatosis were positive to this marker indicating complete lack of specificity. Moreover, Goldblum, et al. [6] reported that nuclear beta catenin gives much background nonspecific staining and so is difficult for interpretation.
Carlson Jw and Fletcher CD [8] found less sensitivity for Nuclear beta-catenin in desmoid fibromatosis as 24 out of their studied 30 cases were positive (80%). They also reported positive expression in low-grade myofibroblastic sarcomas (30%), solitary fibrous tumors (22%) and GIST (5%). They also studied other spindle cell lesions as neurofibromas, schwannomas, nodular fasciitis, leiomyosarcomas, inflammatory myofibroblastic tumours, fibromas of tendon sheath and Gardner fibromas and found total negative stain for this marker in all these mimic spindle cell lesions. They concluded that positive stain for nuclear beta catenin is suggestive but not definitive for desmoid tumor and negative stain doesn't exclude its diagnosis.
In the present study, Calretinin immunohistochemical expression was positive 85.1% of cases of deep fibromatosis (48.1% patchy and 37% diffuse) (7.4% nuclear, 77.8% cytoplasmic and nuclear). Our results were concordant to Andino L, et al. [18] who found calretinin expression in 75% (44/58) of desmoid fibromatosis and Barak S, et al. [15] who studied the expression of calretinin in desmoid tumor and other spindle cell mimic lesions. They found 75% with 44 out of their 58 studied cases of desmoids fibromatosis were positive.
In the present work; calretinin showed focal nuclear staining in 83.3% of schwannoma and diffuse cytoplasmic with patchy nuclear expression in 33.3% of LMS. In a study by Park, et al. [19], calretinin was found in 26.7% of the schwannomas (27/101), but it was not found in any of the neurofibromas. They concluded that calretinin can be used as a highly specific marker for diagnosis of schwannoma when put in differential diagnosis with neurofibroma. Very few studies in literature revealed positive expression of calretinin in LMS. Focal positive calretinin staining was reported in 11/28 LMS cases in one study Shah, et al. [20] and in 2 out of 10 cases of pleomorphic LMS in another study Cates, et al. [21]. While Mittenen, et al. [22] found negative calretinin in all LMS cases. This finding needs larger studies for evaluation of its significance.
All cases in our study (100%) of MPNST, synovial sarcoma, SFT and GIST were negative for Calretinin. Contrary to our results; Mittenen, et al. [22] found fields positive for tumor cells in 71% of 41 biphasic synovial sarcomas, 52% of monophasic synovial sarcoma and 56% of poorly differentiated synovial sarcomas. Also 2 or 15 MPNST showed focal reactivity. But concordant to our data all cases of GIST were negative in their series. We suggest that the small sample size (5 cases synovial sarcoma) in the present study may contribute to this difference.
Sensitivity of calretinin in our study was 85.2% and specificity 80.9% with positive predictive value 59% and negative predictive value 94%. However Barak S, et al. [15] reported lower specificity because of the different spindle cell lesions included in their study where 50% (21/42) of proliferative fasciitis, 23% (8/35) of nodular fasciitis, 33% (13/40) of benign fibrous histiocytoma, 35% (22/62) of malignant fibrous histiocytoma, and 13% (4/31) of solitary fibrous tumors. They concluded that calretinin expression is fairly common in benign and malignant fibroblastic and myofibroblastic lesions.
We noted that the pattern of expression of calretinin in deep fibromatosis is variable from case to case as 48.1% of cases showed patchy expression which could be the reason of lowering sensitivity and may give false negative results in tru cut biopsies while 37% diffuse. It was nuclear in 7.4% but cytoplasmic and nuclear in 77.8%.
We recommend further studies on a larger scale for validating the diagnostic utility of calretinin versus other spindle cell mimic lesions, especially on lesions with debatable results in literature like LMS and synovial sarcoma.
From this study, we conclude that calretinin can raise the diagnostic accuracy of deep fibromatosis when put in differential diagnosis with other spindle cell lesions but should be used with the other immunehistochemical panel. Also, it can have a diagnostic utility in schwannoma.
No conflict of interest.
None.
None.