Fibrous dysplasia (FD) is a benign neoplasm of bone which is primarily characterized by the inability of bone-forming tissue to produce mature lamellar bone, instead arresting as woven bone[1]. FD may be present in one bone (monostotic) or have multiple foci (polyostotic) [1,2]. Additionally, FD lesions may occur alone or as a part of broader diseases such as McCune-Allbright syndrome (MAS) and Mazabraud's syndrome [[1,3-5]. The primary cause of FD are mutations in the GNAS gene which encodes the Gsα subunit of a stimulatory G protein [6]. These mutations disrupt the activity of the G protein pathways and result in abnormalities in the proliferation and differentiation of skeletal stem cells into mature osteocytes capable of producing lamellar bone [2,7]. The most common GNAS mutation is a single-nucleotide mutation on codon 201 resulting in the substitution of cysteine, histidine, or serine for arginine [6]. The mutant form of GNAS/ Gsα is referred to as the gsp oncogene, which is implicated in the endocrine tumors associated with MAS and soft tissue myxomas in Mazabraud's syndrome, as well as various malignant neoplasms in the brain, thyroid, lung, and pancreas [1,6,8]. Malignancy arising from the FD lesions themselves is rare with osteosarcoma being the most common transformation [9]. However, the presence of FD has been attributed to an over threefold increase in a person's likelihood of developing breast cancer at a younger age in a 2018 study observing a cohort of 255 US American and Dutch women [10]. In this series, all of the women diagnosed with FD and breast cancer had polyostotic FD and most (60%) also had MAS. Additionally, in over 40% of the study population, the breast tumors were positive for the same GNAS mutation as the patients’ FD. In none of the previously reported GNAS positive breast carcinomas were the tumors negative for estrogen receptor, progesterone receptor and HER2 [10]. Herein we report the first case of triple negative invasive ductal carcinoma in a patient with polyostotic FD.
Fibrous dysplasia, GNAS mutation, Triple negative breast cancer
A 55-year-old woman presented to our tertiary care facility referred from an outside institution after a 3 cm mass in her left breast was identified (Figure 1). The mass was biopsied, and she was subsequently diagnosed with a high grade invasive ductal carcinoma (IDC) (Figure 2). The cancer was negative for estrogen receptor, progesterone receptor and Her2. Additionally, the proliferative rate as assessed by Ki-67 was 85 percent. An axillary lymph node was suspicious by imaging, but not biopsied. A nuclear medicine bone scan with a Tc-99 m radiotracer was used to stage her cancer (Figure 3). The imaging showed increased radiotracer uptake in the right proximal femur and right distal tibia suggestive of metastases and therefore, the patient was considered to have stage IV metastatic breast carcinoma. However, radiographs of the right femur and tibia showed stable lesions without aggressive cortical involvement and a ground glass appearance suggestive of fibrous dysplasia (Figure 4A and 4B). A subsequent biopsy of the proximal femur lesion showed atypical fibrous tissue with S-shaped woven bone trabeculae that tested negative for MDM2 amplification, essentially ruling out low grade osteosarcoma which can sometimes histologically mimic FD [11] and confirming the imaging impression of fibrous dysplasia (Figure 5). Additional genetic testing showed that the fibrous dysplasia was caused by a somatic mutation on codon 201 of the GNAS gene, substituting cytosine in CGT for thymine (TGT). As the fibrous dysplasia was found incidentally as part of the patient’s metastatic work up and was generally asymptomatic, it was clinically followed. Her triple negative breast cancer responded to preoperative chemotherapy, and a subsequent left breast segmental mastectomy showed only treatment related changes and no residual disease. The sentinel lymph nodes were negative for metastatic carcinoma. The breast specimen was evaluated by molecular sequence analysis which revealed no mutation in exon 8 or 9 of the GNAS gene. The patient is currently well with stable polyostotic fibrous dysplasia and without residual carcinoma.
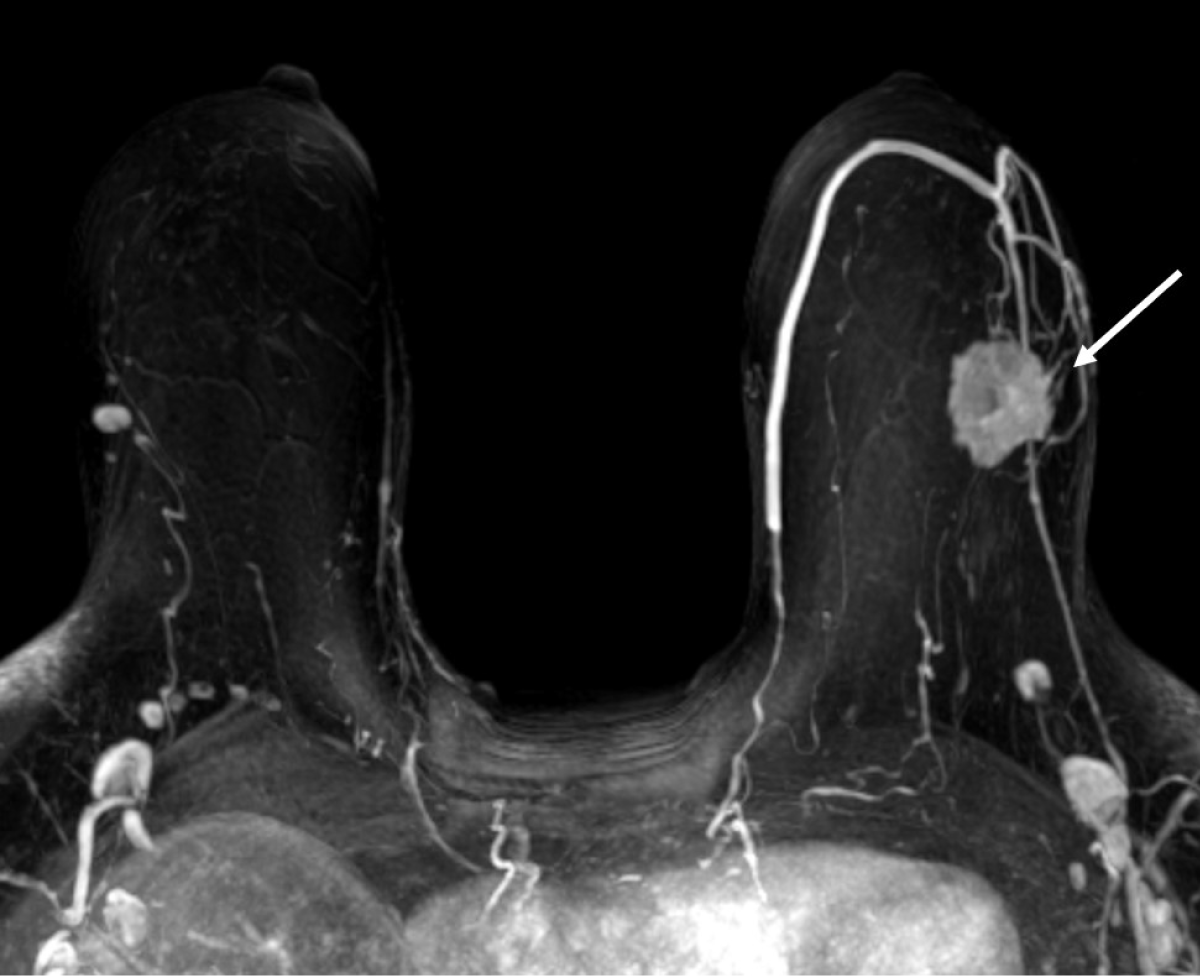 Figure 1: Maximum intensity projection MR image shows a 3 cm irregular enhancing mass in the upper outer quadrant of the left breast (arrow).
View Figure 1
Figure 1: Maximum intensity projection MR image shows a 3 cm irregular enhancing mass in the upper outer quadrant of the left breast (arrow).
View Figure 1
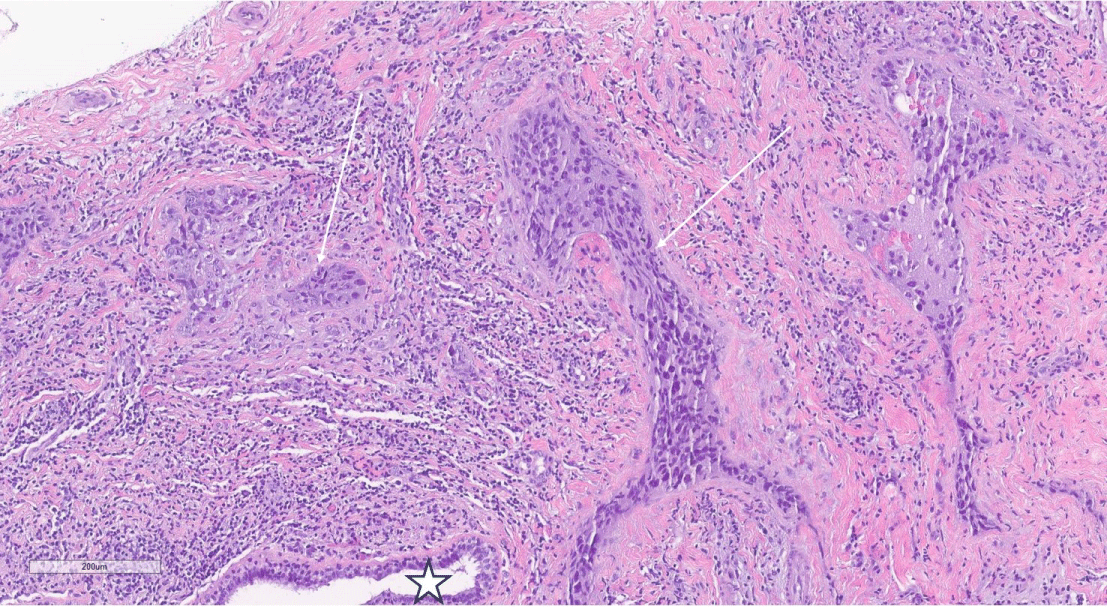 Figure 2: H&E, 10X magnification of breast core biopsy showing both a normal duct (star) adjacent to grade 3 invasive ductal carcinoma (arrows).
View Figure 2
Figure 2: H&E, 10X magnification of breast core biopsy showing both a normal duct (star) adjacent to grade 3 invasive ductal carcinoma (arrows).
View Figure 2
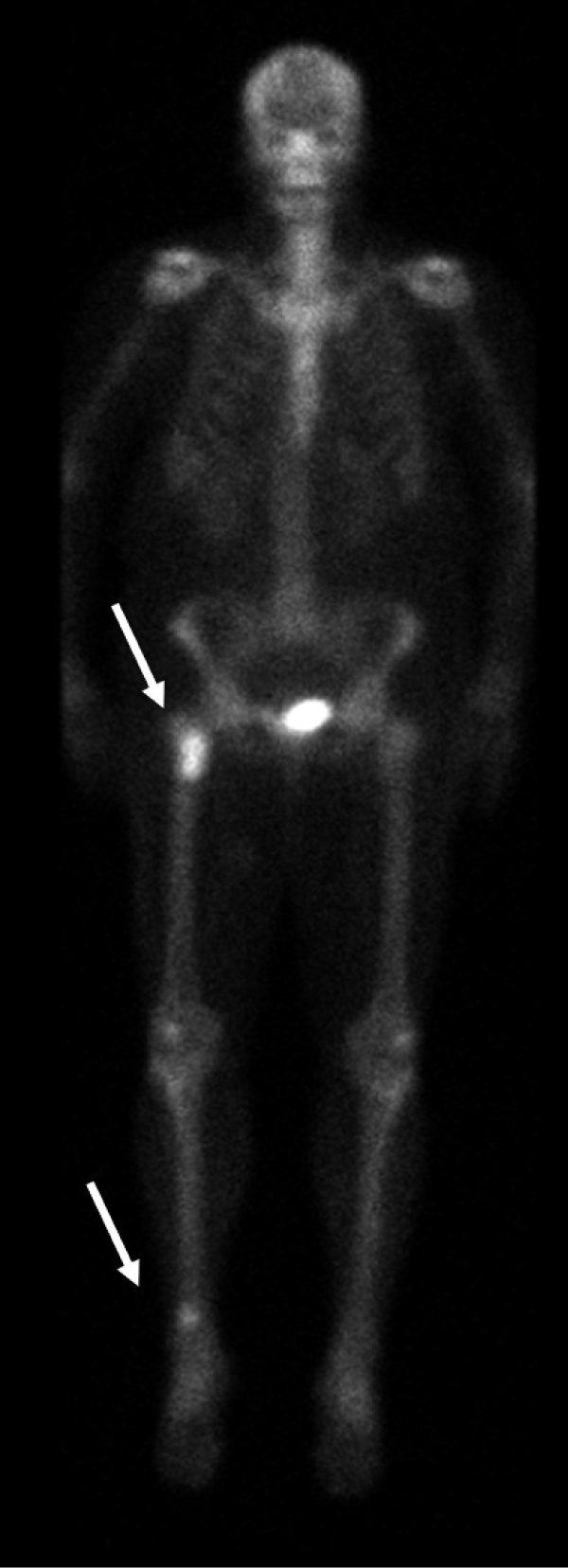 Figure 3: Planar whole-body scintigraphy shows focal accumulation of the radiotracer in the proximal right femur and distal region of the right tibia (arrows).
View Figure 3
Figure 3: Planar whole-body scintigraphy shows focal accumulation of the radiotracer in the proximal right femur and distal region of the right tibia (arrows).
View Figure 3
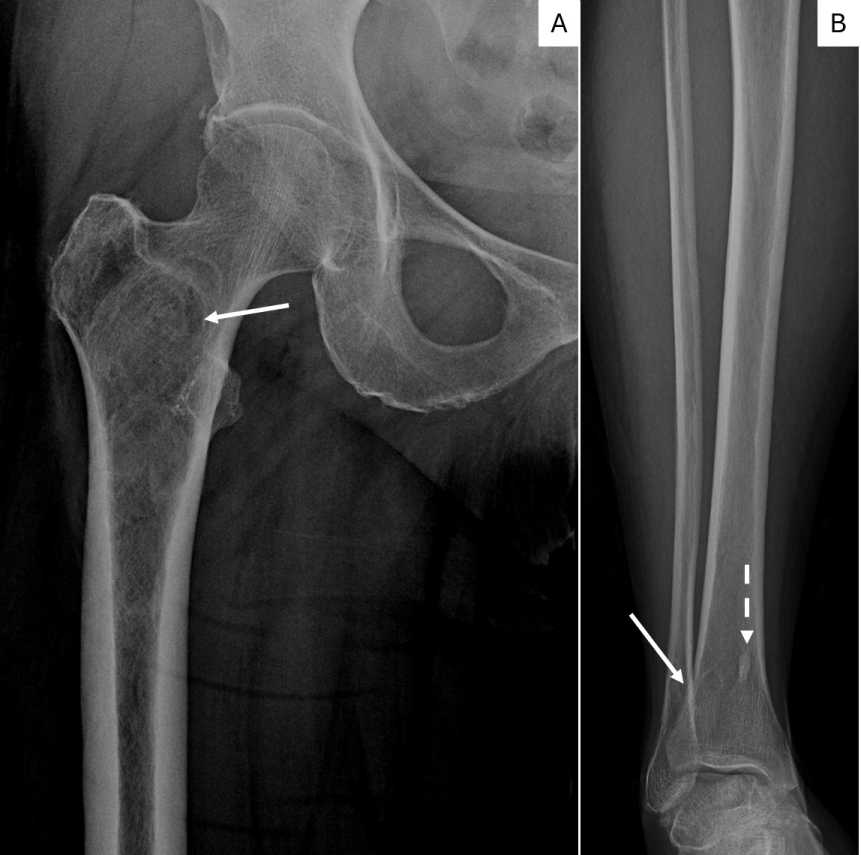 Figure 4: Figure 4a. X-ray of the right femur shows a intramedullary intertrochanteric/subtrochanteric lesion in the right femur with minimal expansion of proximal diaphysis without aggressive cortical involvement. Sclerotic rim, minimal expansion and ground glass appearance suggest Fibrous Dysplasia. (arrow).
Figure 4: Figure 4a. X-ray of the right femur shows a intramedullary intertrochanteric/subtrochanteric lesion in the right femur with minimal expansion of proximal diaphysis without aggressive cortical involvement. Sclerotic rim, minimal expansion and ground glass appearance suggest Fibrous Dysplasia. (arrow).
4b. X-ray of the tibia shows a metaphyseal osteolytic lesion with smooth to sclerotic rim of FD in right distal tibia superimposed with normal trabeculae (white arrow). A bone island is also present in the medial tibia (dashed arrow).
View Figure 4
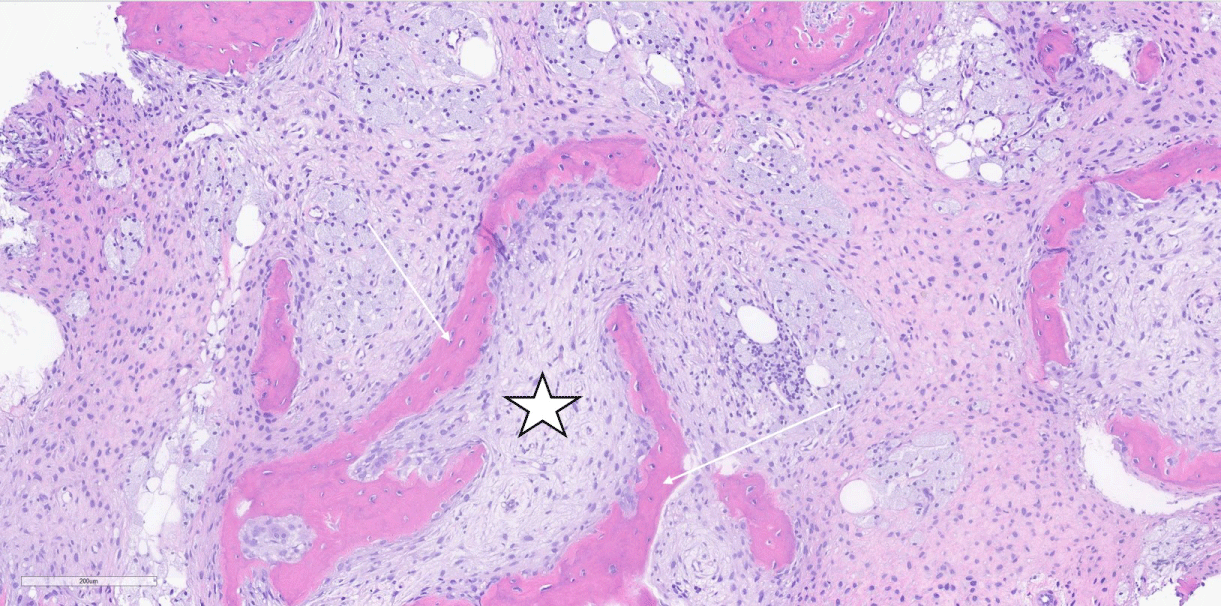 Figure 5: H&E, 10 X magnification of femur core biopsy showing fibrous dysplasia demonstrating abnormal proliferation of stellate fibroblasts (star) interspersed between woven bone with absence of osteoblastic rimming (arrows).
View Figure 5
Figure 5: H&E, 10 X magnification of femur core biopsy showing fibrous dysplasia demonstrating abnormal proliferation of stellate fibroblasts (star) interspersed between woven bone with absence of osteoblastic rimming (arrows).
View Figure 5
GNAS mutations have been linked to oncogenesis not only in osteogenic progenitor cells, but also cells derived from ductal, epithelial and mesenchymal cells. Notably, GNAS codon 201 mutations have been demonstrated to alter pre-invasive pancreatic lesions in mice, progressing to predominantly well-differentiated invasive carcinoma [12]. While the role of GNAS mutations in the onset of FD has been extensively studied, little research has been done to see if abnormal GNAS mediated differentiation of mesenchymal stem cells can give rise to breast cancer. The GNAS mutation is rarely reported in breast cancer [10] but not triple negative breast cancer. Moreover, there are very few reports of polyostotic FD mimicking bone metastasis in the literature.
This 55-year-old patient presented with an extremely rare case of triple negative breast cancer and polyostotic FD mimicking multiple bony metastasis. Triple negative breast cancers are uncommon, comprising 10 to 15% of all mammary carcinomas [13], and can be associated with genetic mutations, notably BRCA1 and p53. While the breast lesion was identified first in this patient, it can be reasonably posited that the breast carcinoma was preceded by the FD due to the high proliferation rate in the breast cancer and radiographic evidence of calcifications and slow ballooning of the femur around the lesion, usually indicative of a long-standing lesion. Her FD lesions were likely caused by somatic GNAS mutations which led to the abnormal proliferation of immature woven bone with no lamellar structure.
While fibrous dysplasia is a benign disease, there is a clear association between the GNAS gene involved in fibrous dysplasia and an increased risk of developing malignancies such as osteosarcoma [9] as well as being diagnosed with breast cancer at a younger age [10]. Additionally, genetic testing of patients with FD and breast cancer has previously shown in the same mutation on GNAS codon 201 in 44% of patients studied [10]. This association suggests a correlation between the mutations and the development of early onset breast cancer.
This case study illustrates the importance of testing additional tumors in patients with fibrous dysplasia. This particular patient’s breast cancer was negative for the GNAS mutation; however, the overall coincidence rate of GNAS in breast cancer in women with FD is over 40% [10]. Molecular targeted therapy could be further investigated as a treatment option for patients with GNAS mutated breast cancers to inhibit the specific pathways that result in GNAS mediated tumorigenesis.
Unfortunately, the GNAS gene has historically been viewed as un-targetable in part due to the intracellular location of the gene resulting in poor drug binding sites [6]. However, there have been recent advances in personalized medicine with CRISPR gene editing showing promise in an in vitro setting [14]. Protein kinase inhibitors such as MEK inhibitors, which inhibit components of the MAPK pathway and therefore curtail cell proliferation, are also being investigated [15]. This case report describes a patient with triple negative breast cancer and FD and illustrates the salient radiographic and histologic features of FD which should be included in the differential diagnosis of patients with cancers known to metastasize to bone.
Benjamin H Perkins- wrote original draft and edited manuscript.
John E Madewell MD- Writing, reviewing and editing of manuscript. Read and approved manuscript contents before submission.
Nina Tamirisa MD- Writing, reviewing and editing of manuscript. Read and approved manuscript contents before submission.
Beatriz E Adrada MD- Writing, reviewing and editing of manuscript. Read and approved manuscript contents before submission.
Wendong Yu MD- Writing, reviewing and editing of manuscript. Read and approved manuscript contents before submission.
Lavinia P Middleton MD- Conceptualization. Writing, reviewing and editing of manuscript. Read and approved manuscript contents before submission. Supervision.
All authors read and approved the manuscripts contents before final submission.
Beyond the primary affiliations, there was no additional support provided for this research.
None
During the preparation of this work the authors used Open Evidence in order to assist in the literature review process. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.