Purpose: To assess denture adhesive (DA) efficacy in preventing gingival abrasion from infiltrating food particles.
Materials and Methods: This was a 2-treatment, 2-period, examiner-blind, crossover, randomized, controlled pilot clinical trial involving 10 adults with a full maxillary and/or mandibular denture opposed by natural or artificial dentition and a history of food particle infiltration. For each 3-day treatment period, participants were assigned to use an optimized calcium/zinc partial salt of polyvinyl methyl ether/maleic acid (Fixodent Professional) DA (applied in a continuous pattern) or no adhesive. Participants consumed four 20-g peanut servings. Before the peanut challenge, gingival abrasions were stained and assessed using the Danser Abrasion Index by a trained examiner. Statistical tests were 2-sided with a 10% type I error rate.
Results: The DA was significantly more effective than no adhesive at reducing the number and size of gingival abrasions after first use with 1.68 versus 4.10 mean number of abrasions, respectively (59.0% fewer with DA; p = 0.036) and a mean lesion size of 4.91 mm2 versus 21.77 mm2, respectively (77.5% smaller with DA; p = 0.031). This benefit persisted across days 2 and 3 with 1.58 versus 4.25 mean number of abrasions, respectively (62.8% fewer with DA; p = 0.007) and a mean lesion size of 4.15 mm2 versus 26.00 mm2 (84.1% smaller with DA; p = 0.044). Average chewing duration was 268.0 seconds with DA and 366.6 seconds without DA.
Conclusion: The DA was significantly more efficacious than no adhesive at preventing food-related gingival lesions in denture wearers.
Denture, PVM MA copolymer, Adhesive, Methods
The population of denture wearers is significant, with an international study finding 8% of adults wear complete dentures [1]. Dentures offer a wide range of esthetic, functional, and emotional benefits for edentulous patients. However, some denture wearers report avoiding certain foods due to perceived compromised biting and chewing function [2]. A recent systematic review reported that edentulous patients who wear full dentures are at risk for reduced eating function, inadequate fruit and vegetable intake, and poorer eating-related quality of life compared to the fully dentate population [3]. Another nutrition-related hurdle posed by dentures is the infiltration of food particles next to the oral mucosa, which can promote gingival discomfort and irritation [4]. Indeed, denture wearers report that food infiltration is their biggest concern and causes them to avoid eating foods that are likely to get stuck in or under their denture [5].
Dental professionals are uniquely poised to understand and address these potential denture-associated complications. Multiple studies have shown that denture adhesive (DA) can improve both bite force and masticatory performance [6-9]. This benefit extends even to notoriously difficult-to-chew foods, such as carrots, raisins, and meat [10], and offers denture wearers the possibility of a more varied and nourishing diet [11]. Research has also shown that denture adhesives can help reduce food infiltration [12-14] and promote comfortable denture wear [4,15]. There has been little research, however, specifically assessing the benefit of DA in preventing gingival abrasions caused by food infiltration.
This pilot clinical study evaluated the efficacy of a DA in preventing gingival abrasion following a peanut-eating challenge in complete-denture wearers. The primary end point was mean abrasion size over days 2 and 3 of a 3-day study period. The secondary end point was mean number of abrasions over days 2 and 3 of a 3-day study period.
The study protocol and procedures were reviewed and approved by the U.S Investigational Review Board, Inc. in Miami, FL, USA (U.S.IRB 2019SRI/07). All participants provided written, informed consent and signed an image release form on the first day of the study.
This was a single-center, 2-treatment, 2-period crossover study. The objective of this study was to evaluate a DA with respect to its ability to prevent gingival abrasions in the context of a peanut-eating challenge. The primary end point was mean abrasion size over days 2 and 3 of a 3-day study period. The secondary end point was mean number of abrasions over days 2 and 3 of a 3-day study period. Additionally, the size and number of abrasions after first use (day 2) were also investigated.
Participants were recruited by Salus Research and the study visits occurred at the Salus clinical research site in Fort Wayne, Indiana, USA from November to December 2019. The study participants were healthy adults, each with a full maxillary denture, a full mandibular denture, or both, with dentures opposed by either natural or artificial dentition. All participants had a history of food particle entrapment beneath their dentures and resultant gingival irritation. Enrolled participants agreed to follow their assigned study regimen of adhesive use or nonuse during the respective study periods, to abstain from eating for at least 4 hours prior to the challenge visits, and to eat 20 g peanuts 4 consecutive times a day for each peanut challenge. Enrolled participants also agreed to refrain from the use of non-study DA on study visit days, to refrain from any adhesive use between visit days during the “no adhesive” period of the study, and to refrain from participating in the study of any other oral or dental product for the duration of the current study.
Participants were excluded on the basis of allergy to study materials, insulin dependence, or any disease or condition that was expected to interfere with the safe participation in study procedures or that was expected to increase susceptibility to gingival abrasion (e.g., diabetes or xerostomia). Participation would be discontinued (or a participant’s data excluded from analysis) on the basis of noncompliance with study procedures, intolerance to study procedures, the advent of insulin dependence or any other health condition that might interfere with study procedures or that might increase susceptibility to gingival abrasion, or the alteration of a participant’s dentures during the study periods.
In this study, an optimized calcium/zinc [Ca/Zn] partial salt of polyvinyl methyl ether/maleic acid [PVM-MA] adhesive (Fixodent Professional), which has a thin nozzle to facilitate a continuous application pattern, was compared with a negative control (no adhesive). During a participant’s “adhesive” period, DA was applied by a trained dental professional using a continuous pattern (See Figure 1), in accordance with manufacturer’s instructions, after the denture was rinsed and dried. For each participant, the applied product weight was recorded after the first application, and subsequent applications reproduced the same mass of applied product, ± 0.02 g. After each product application, participants seated their own dentures. Participants were provided with denture brushes with which to clean their dentures at home.
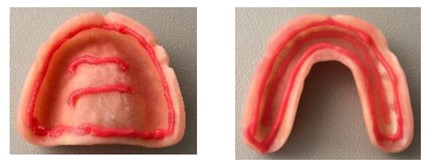 Figure 1: Denture adhesive continuous application pattern.
View Figure 1
Figure 1: Denture adhesive continuous application pattern.
View Figure 1
On period 1, day 1, all qualifying participants provided demographic and medical history information. A computer-generated randomization schedule provided by the sponsor was used to randomly assign participants to a treatment sequence (AB or BA, where “A” represents adhesive and “B” represents no adhesive). Sequence assignment and test product application were performed by study personnel not associated with the examination and occurred in a protected area to ensure the blinding of the examiner.
Participants arrived at each visit with no adhesive on their dentures. Each visit began with denture cleaning, and an oral soft and hard tissue exam of the oral cavity and perioral area followed by a professional gingival abrasion evaluation, treatment administration (adhesive or no adhesive), and peanut challenge.
For each peanut challenge, each participant ate four 20-gram servings of peanuts (total, 80 g). The peanut challenge was timed, and a note was made if a participant was unable to eat the entire serving of peanuts during the allotted time. During the challenge, participants could take breaks as needed, and small sips of water were permitted. Following the challenge, participants were dismissed for the day and instructed to continue wearing the adhesive until bedtime, at which time they were to remove the adhesive with warm water and a denture brush. Figure 2 depicts the visit flow for the test period.
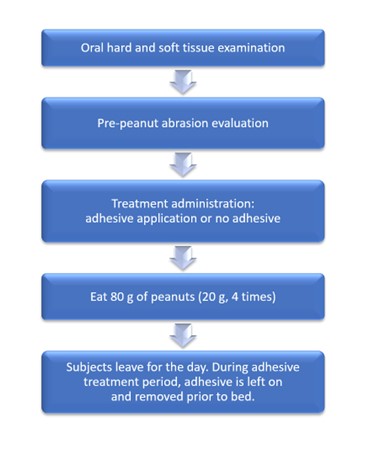 Figure 2: Visit flow on each day of treatment period.
View Figure 2
Figure 2: Visit flow on each day of treatment period.
View Figure 2
On day 2 and day 3, continuance criteria were evaluated and the experimental procedure (from denture cleaning through dismissal and final instructions) was repeated. There was a washout period of at least 2 weeks between periods 1 and 2, after which all participants completed an accountability report. During the washout period, participants were permitted to wear DA if it had been their habit to do so before the study.
Participants’ gingivae were evaluated by a trained and calibrated dental professional examiner with respect to the size (in millimeters 2 ) and number of abrasions, using an adaption of the Danser Abrasion Index [16], according to the following procedure.
For each participant, oral mucosa was dried with the air blast and then the participant swished for 10 seconds with 5 ml Mira-2-Ton dye, (Hager & Werken, Germany), followed by swishing for 10 seconds with water. Next, mucosa under the denture was dried with the air blast. Each quadrant was assessed for abrasions. The location of each abrasion (now stained blue) was noted, and the length and width of each abrasion were measured with a periodontal probe (Michigan O Single End Probe with Williams Markings). Loosely attached discolorations were excluded from the evaluation. If an area of questionable discoloration could not be removed, it was recorded as an abrasion (vs plaque). The locations and shapes of all abrasions were recorded on maxillary and mandibular ridge charts (see Figure 3).
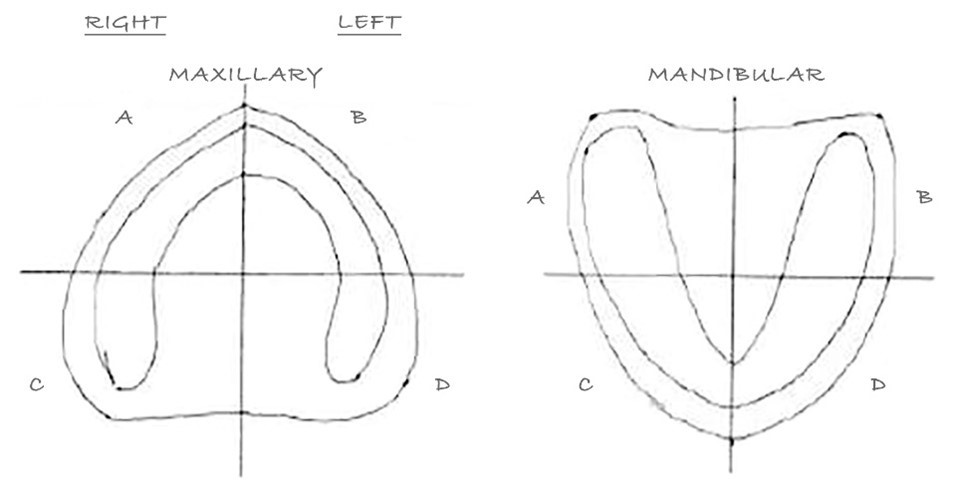 Figure 3: Denture ridge chart to document gingival abrasion characteristics per quadrant.
View Figure 3
Figure 3: Denture ridge chart to document gingival abrasion characteristics per quadrant.
View Figure 3
The primary null hypothesis was that there was no between-treatment difference in average pre-challenge abrasion size assessed over days 2 and 3 after adjusting for average baseline abrasion size. The secondary null hypothesis was that there was no between-treatment difference in average number of abrasions assessed over days 2 and 3 after adjusting for average baseline abrasion number. The sample size (10 participants) for this pilot study was chosen for logistical reasons.
Demographic and baseline clinical data were summarized for all randomized participants. The average size and average number of pre-challenge abrasions across days 2 and 3 were assessed by a repeated measures analysis of covariance for a crossover design. Day, treatment, period, and baseline abrasion size or number were included as covariates; participant and participant by period interaction were included as a random effect. Average duration of chewing time across days was also analyzed for treatment differences using the same crossover analysis. All statistical tests were 2-sided with a 10% type I error rate.
50 participants were screened, and 10 participants were enrolled in the study. All participants had complete maxillary and mandibular dentures. Nine participants completed all days of both study periods; 1 participant completed the first 2 days of the first study period and then voluntarily withdrew from the study. There was no statistically significant difference at baseline (pre-food challenge) between study periods with respect to the number of lesions or the size of lesions. Demographic and baseline data are summarized in table 1 and table 2, respectively.
Table 1: Baseline demographic data. View Table 1
Table 2: Baseline pre-food challenge results: lesion number and size.a View Table 2
At the beginning of study day 2 (following the food challenge on day 1), oral examination revealed that adhesive use resulted in statistically significantly fewer lesions than no adhesive, with a mean of 1.68 vs 4.10, respectively (p = 0.036), representing a 59.0% reduction in the average number of lesions. Adhesive use also resulted in statistically significantly smaller lesions than no adhesive, with a mean size of 4.91 mm 2 vs 21.77 mm 2 , respectively (p = 0.031), representing a 77.5% reduction in average lesion size (Table 3 and Figure 4).
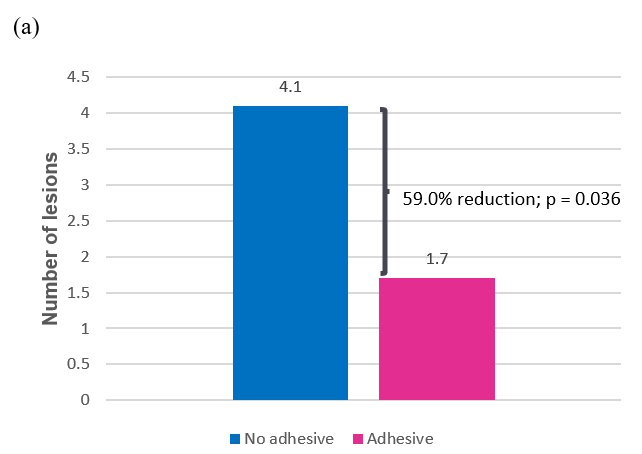 Figure 4a: Average number of lesions after first use (day 2);
View Figure 4a
Figure 4a: Average number of lesions after first use (day 2);
View Figure 4a
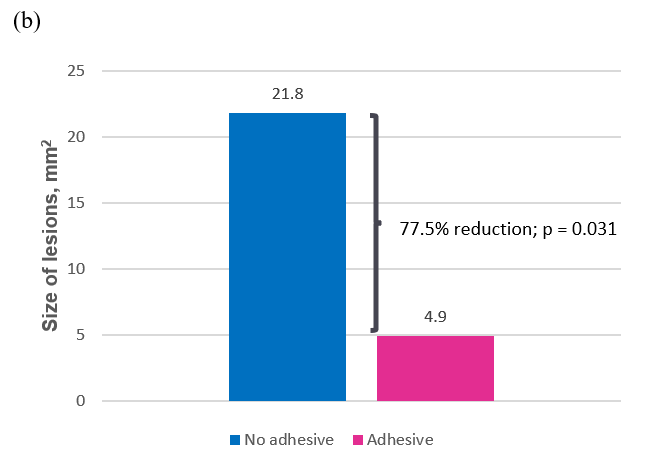 Figure 4b: Average size (mm2) of lesions after first use (day 2).
View Figure 4b
Figure 4b: Average size (mm2) of lesions after first use (day 2).
View Figure 4b
Table 3: Lesion efficacy results. View Table 3
Similar results were seen across days 2 and 3, with adhesive use producing statistically significantly fewer lesions (mean, 1.58 vs 4.25; p = 0.007) and smaller lesions than no adhesive (mean, 4.15 mm 2 vs 26.00 mm 2 ; p = 0.044). These differences represent a 62.8% reduction in the average number of lesions and an 84.1% reduction in average lesion size for adhesive use compared to no adhesive (Table 3 and Figure 5).
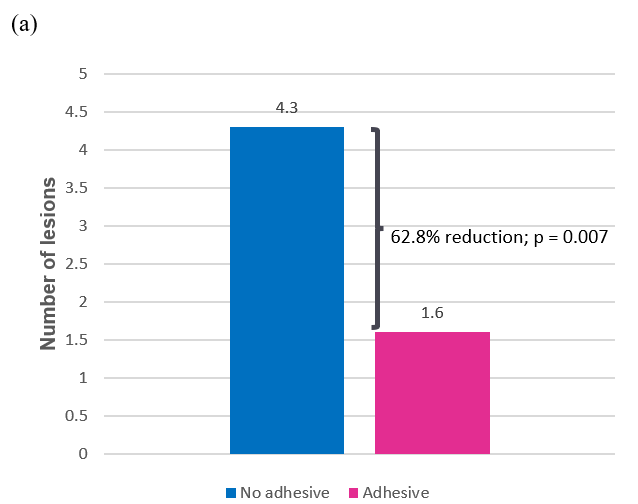 Figure 5a: Average number lesions across days 2 and 3;
View Figure 5a
Figure 5a: Average number lesions across days 2 and 3;
View Figure 5a
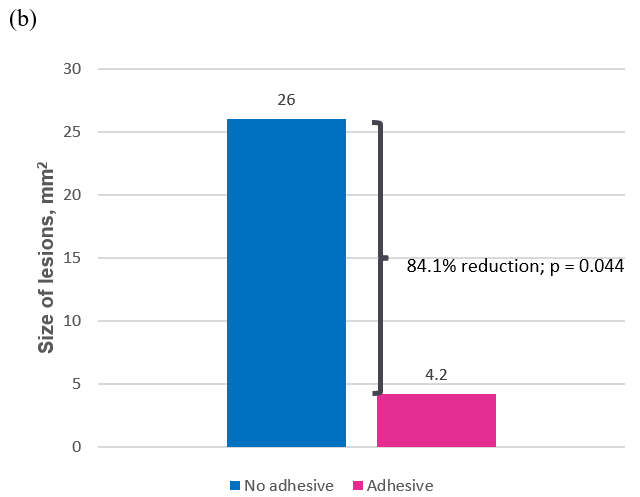 Figure 5b: Average size of lesions (mm2) across days 2 and 3.
View Figure 5b
Figure 5b: Average size of lesions (mm2) across days 2 and 3.
View Figure 5b
The average chewing duration time across days 1-3 was 268.0 seconds with adhesive and 366.6 seconds without adhesive across the 4 repeat peanut challenges on each day. This represents approximately 26% less chewing time when adhesive was used.
The results of this study show that an optimized Ca/Zn DA, when applied in a continuous application pattern and subjected to a peanut-eating challenge, significantly reduced mucosal abrasions under complete dentures across 2 days of use, relative to the abrasions associated with no adhesive use (Table 3). There was a 62.8% reduction in the number of abrasions and an 84.1% reduction in abrasion size with adhesive use versus no adhesive. Additionally, the use of adhesive resulted in less time spent chewing during the peanut challenge compared to no adhesive use.
These findings are consistent with those of prior research, which showed that DA significantly reduces food infiltration under dentures [12-14]. Tarbet, et al. found significant improvements in complete denture wearers’ subjective ratings for food infiltration with four different foods when study participants used adhesive versus no adhesive [12]. Patel and colleagues found a reduction in food infiltration among complete denture wearers with adhesive use based on objective and subjective assessments [13]. Muñoz-Viveros, et al. showed objective and subjective improvements in food infiltration among well-fitting removable partial denture wearers who used denture adhesive [14]. Findings from the latter two trials [13,14] also demonstrated that a food infiltration benefit for denture adhesive is not limited to poor-fitting dentures, as study participants in both trials had fair to well-fitting dentures and experienced a significant reduction in food particles under their denture when using adhesive.
This study design, which assessed the size and number of gingival lesions following a replicate challenge of chewing peanuts, represents a novel procedure for assessing DA efficacy with respect to the prevention of abrasions due to food particle infiltration. This is an area with limited evidence in the scientific literature. Strengths of the study include use of a randomized controlled crossover design, which enabled each subject to be their own control when comparing adhesive to no adhesive, and blinding of the examiner to the treatment assignment. There were some limitations, including a small base size and inclusion of only one food type. Future research could expand upon these learnings by increasing the base size, evaluating additional abrasive foods, and assessing partial dentures.
These findings add to the body of research that suggests DAs can improve nutrition among denture wearers. Not only do DAs improve the ability of users to bite and chew with well-retained, stable dentures [6-10], DAs can also be a solution to the uncomfortable and potentially injurious retention of food particles [4,12-15]. Both of these attributes can free denture wearers from a restricted diet and allow them to enjoy a nourishing diet with confidence and comfort. In light of these results, dental professionals should consider use of DAs to enhance the well-being of their denture-wearing patients.
The experimental DA effectively prevented gingival abrasions due to infiltrating food particles under complete dentures. The randomized controlled design of the present study and its exclusive enrollment of participants with a history of food particle entrapment recommend its results as worthy of confidence. These results could form the basis of future investigation, expanded to include a larger study population and wearers of partial dentures.
The authors thank Marisa DeNoble Loeffler, MS, for medical writing assistance in the preparation of the manuscript.
Dr. Klukowska and Dr. Grender are employees of The Procter & Gamble Company. Dr. J. Milleman and Dr. K. Milleman report no conflicts of interest.
The study was funded by The Procter & Gamble Company, Cincinnati, Ohio, USA. Medical writing assistance was funded by The Procter & Gamble Company.