Currently there remains controversy in the surgical management of rhegmatogenous retinal detachment (RRD) due to giant retinal tears (GRTs), a potentially blinding condition. To clarify which surgical technique is better depending on the origin and magnitude of the giant tear this study aimed to analyze the anatomic and functional outcomes. To analyze trans- and postoperative surgical complications, we used long-term final postoperative structural, optical coherence tomography (OCT) and correlated the results with the final postoperative best-corrected visual acuity (BCVA) in three different groups of eyes.
A long-term, comparative, retrospective, consecutive case series on seventy-six eyes of 66 patients that were recruited and classified according to the degree of GRT-associated RRD extension as follows: group 1 (n = 42 eyes) with GRT-associated RRD extension < 180°; group 2 (n = 23 eyes) with GRT-associated RRD extension = 180°-270°; and group 3 (n = 11 eyes) with GRT-associated RRD extension > 270°. Structural and functional outcomes were compared across groups.
Of the 76 eyes analyzed, 63 were phakic, and 13 were pseudophakic. The mean age of the patients was 43.0 ± 13.0 years (range, 19-76 years); 36 females, and 40 males. The mean preoperative time for GRT surgery was 1.8 weeks, the mean preoperative and postoperative BCVA was 1.87 logMAR and 0.35 logMAR, respectively (p < 0.05), and the mean postoperative follow-up was 28.1 months. Five patients (6.6%) had bilateral GRT-associated RRD, 61 patients (80.3%) had a monocular condition, and 21 eyes (27.6%) had final BCVA of ≥ 20/40. Proliferative vitreoretinopathy resulted in multiple surgeries in 31.6% of the eyes. Postoperative OCT yielded abnormal retinal thickness, ellipsoid zone (EZ) disruptions, and external limiting membrane (ELM) line discontinuities in all groups, predominantly in GRTs macula off-associated RRD requiring multiple surgeries.
Multiple structural alterations in spectral-domain OCT biomarkers were observed. Eyes that developed secondary epiretinal membrane (ERM) proliferation showed significantly improved BCVA after proliferation and the internal limiting membrane (ILM) was removed. The structural findings correlated with the BCVA allow us to conclude severe consequences of the macular structure and that, despite a fully reattached retina without ERM proliferation, GRTs-associated RRD has a guarded functional prognosis.
Giant retinal tears, Rhegmatogenous retinal detachment, Brilliant blue dye, Epiretinal membrane, Internal limiting membrane, Macula-off giant retinal tear-associated rhegmatogenous retinal detachment, Primary vitrectomy, Scleral buckle
C3F8: Perfluoropropane; BBG: Brilliant Blue G; BCVA: Best-Corrected Visual Acuity; CSFT: Central Subfoveal Thickness; DONFL: Dissociated Optic Nerve Fiber Layer; ELM: External Limiting Membrane; ERM: Epiretinal Membrane; GRT: Giant Retinal Tear; ILM: Internal Limiting Membrane; IS/OS: Internal Segment/External Segment; OCT: Optical Coherence Tomography; PFCL: Perfluorocarbon Liquid; PVD: Posterior Vitreous Detachment; PVR: Proliferative Vitreoretinopathy; RD: Retinal Detachment; RPE: Retinal Pigment Epithelium; RRD: Rhegmatogenous Retinal Detachment; SB: Scleral Buckle; SD: Spectral Domain; SS: Swept Source; SRF: Subretinal Fluid; VIF: Variance Inflation Factors; WAVS: Wide-Angle Viewing Systems; WWOP: White Without Pressure
Rhegmatogenous Retinal Detachment (RRD) associated with giant retinal tears (GRTs) is an acute condition that involves a full-thickness circumferential retinal tear of > 90°, with vitreous detachment, and frequently accompanied by vitreous hemorrhage and subretinal hemorrhage [1,2]. The condition is rare, with an estimated incidence rate being reported between 0.05% and 0.09% per 100,000 people per year, a condition that predominantly occurs in men with an incidence of 72% [1-6], they represent 1.5% of the total RRDs and the average age of disease diagnosis is reported to be at 42 years, with a described bilateral not necessarily simultaneous presentation of 12.8% [2-5].
The pathogenesis involves pathologic vitreous traction on the peripheral retina and is often associated with condensation of the peripheral vitreous and liquefaction of the central vitreous [2]. The retinal tear can occur acutely and in different magnitudes, depending on each eye's substrate and conditions, and it can also break sub-acutely, resulting in tears that gradually progress in a zipper fashion [7]. In some cases, the GRT associated with vitreoretinal traction may be caused by the coalition of multiple horseshoe tears that form posterior to the posterior border of the vitreous base during syneresis or collapse and synchytic phenomena due to acute pathologic contraction of the vitreous; all of the above lead with certain local or systemic risk factors along with idiopathic or secondary conditions to the circumferential rupture of the retina greater than one peripheral quadrant [2,7,8]. According to a large UK-based epidemiological study, approximately 55% of GRTs are idiopathic [1]. Similarly, 25% of GRTs are myopia-associated, 14% are associated with hereditary conditions with defects in type 2 collagen synthesis (e.g., Marfan's, Stickler-Wagner, and Ehrler Danlos syndrome), and 12.3% result from close-eye blunt trauma [1,2,8-10]. Local ocular risk factors for the disease include blunt trauma, high myopia, aphakia, and pseudophakia [8-10].
The management of GRTs is significantly challenging due to the high risk of intra- and postoperative complications and the high rate of recurrent RRDs due to the appearance of proliferative vitreoretinopathy (PVR) that reaches an incidence between 40% and 50% with acute alterations in intraocular pressure due to uveal dysfunctions and a pro-cytokine inflammatory cascade from the blood-retinal barrier [11]. Although there is a relative paucity in published information related to long-term functional outcomes of GRT-associated RRD, the reported anatomic success rate is significantly high and is between 80% and 90%, with the final reattachment rate being at 94%-100% [12,13]. In this context, this study intended to determine the postoperative comparative incidence of PVR and epiretinal membrane (ERM) proliferation over the macula, according to the extent of the tear and to the anatomic preoperative status of the macula, statistically analyze other trans- and postoperative surgical complications, provide long-term final postoperative microstructural optical coherence tomography (OCT) findings, and correlate these results with the final postoperative best-corrected visual acuity (BCVA) in different surgical management methods for GRT-associated RRD.
The present study was conducted by the Retina Specialists Unit at Oftalmologia Integral ABC in Mexico City. The study strictly followed the tenets of the Declaration of Helsinki and was approved by the institutional research ethics committee of the institution. All included patients provided written informed consent to access clinical charts for retrospective data analysis. The patient cohort included consecutively enrolled patients diagnosed with GRT-associated RRD who were surgically managed between January 2010 and January 2021. Different types of preoperative GRTs are depicted in Figure 1.
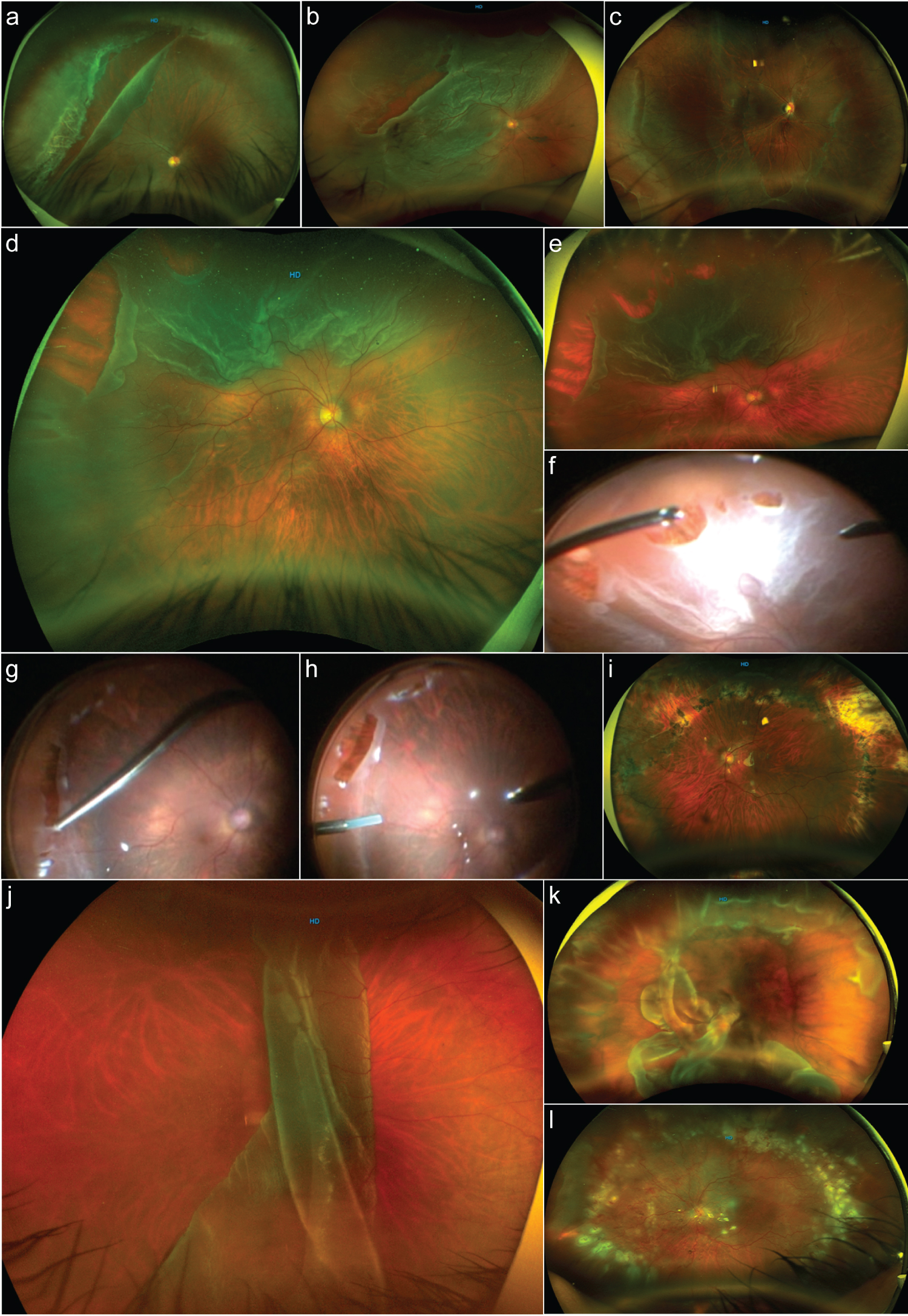 Figure 1: Types of giant retinal tears.
Figure 1: Types of giant retinal tears.
a: An image depicting a giant retinal tear (GRT) associated rhegmatogenous retinal detachment (RRD) from the 8 to 1 o'clock meridian; the retina and macula remain attached. The GRT is located in the preequatorial zone, the anterior border of the retina has a tear over a well-defined area of lattice degeneration associated with vitreous traction, and the posterior border of the GRT shows some rolled back edges, nasal to the GRT. There is a small retinal tear extension. There are well-defined white without pressure (WWOP) areas of retinal appearance throughout the retina periphery. b: A one-quadrant macula-off GRT-associated RRD with an additional rhegmatogenous lesion over the temporal horn of the tear. c: A GRT, inferiorly located in the presence of macula-on RRD with imminent macula involvement. There are extensive WWOP-associated degeneration areas. d: A partitioning GRT-associated macula-off RRD, the posterior border of the main tear shows proliferative vitreoretinopathy grade b with some retina wrinkling and rolled back posterior edge. e: A more peripheral wide-angle color picture of the previous image depicting more clearly the horse-tears in a zipper-type coalition due to severe vitreoretinal traction. f: Previous case surgical image where peripheral vitreous and fluid-to-fluid endodrainage are performed. g: Endodiathermy marking of the extensive retinal lesions. h: Sequential image showing perfluorocarbon liquid assisted endodrainage. i: An image corresponding to the previous case 3 years postoperatively for GRT-associated RRD. j: A macula-off GRT >180° is folded over posteriorly due to complete posterior vitreous detachment. k: Days after a failed vitrectomy and buckled case with a recurrent posteriorly slipped RRD. l: The previous case that has undergone vitrectomy revision; the retina looks completely reattached, and the eye is silicon oil filled.
View Figure 1
The inclusion criteria were set as follows: patients ≥ 18 years with a diagnosis of GRT-associated RRD, evidence of PVR grade B or less, attached retina at the last follow-up examination visit, postoperative best-corrected visual acuity (BCVA) in the functional range of 20/800 (1.60 logMAR units) or better, absence of intraocular silicone oil in the last follow-up visit, at least 6 months of follow-up duration, and a well-documented structural and functional examinations in the last follow-up assessment. Only eyes in which the retina was successfully reattached for a minimum of 6 months of follow-up after the last vitreoretinal surgical procedure were included in the general dataset. The exclusion criteria included: prior complicated vitreoretinal surgery or intravitreal injections, GRT-associated RRD due to penetrating or perforating open-eye injury, GRT-associated RRD combined with macular hole retinal detachment due to myopic traction maculopathy, postoperative BCVA out of the functional range of 20/2000 (counting fingers @ 2 feet or 2.00 logMAR units) or worse, presence of intraocular silicone oil at the last follow-up evaluation visit, severe grade C posterior PVR or anterior PVR with evidence of recurrent and complicated RRD at the last follow-up visit, or a history of active glaucoma. The additional exclusion criteria were untimely follow-up visits, loss of follow-up, surgery in a non-designated institution, evidence of severe complications (e.g., endophthalmitis, recurrent disease, complicated severe PVR RRD at the last follow-up visit, and refractory corneal opacity).
A detailed general ophthalmic evaluation and preoperative examinations were conducted in all patients. The tests included: BCVA assessment, slit lamp biomicroscopic examination, fundus examination by a panfundoscopic contact lens, and indirect ophthalmoscopy. Snellen's visual acuity was converted to logMAR visual acuity for statistical comparison. Axial lengths were measured using partial coherence laser interferometry (Zeiss IOL Master 700; Carl Zeiss Meditec AG, Oberkochen, Germany). The presence of GRT-associated RRD was confirmed by indirect ophthalmoscopy and B-scan ultrasonography (A and B Ultrasound Unit, Quantel Medical, Du Bois Loli, Auvergne, France). A long-term postoperative cross-sectional microstructural evaluation was performed using spectral domain (SD)-OCT Spectralis, SD-OCT RTVue-XR platform, and a swept-source (SS)-OCT device (Topcon Medical Systems, Inc., Oakland, NJ, USA). All OCT images were analyzed by two experienced retina specialists (co-authors) from the participating institution. We used the terminology proposed by the International Nomenclature for Optical Coherence Tomography Panel report to describe the structural postoperative SD-OCT findings [14].
Phacoemulsification with in-the-bag intraocular lens implantation techniques was uneventfully performed in all phakic eyes. A standard 23- or 25-gauge 3-port pars plana vitrectomy (Alcon Constellation Vision System, Alcon Labs, Fort Worth, TX, USA) was performed in all eyes under local anesthesia and sedation by one of the authors (MAQR). The vitrectomy was performed using a contact wide-angle viewing precorneal lens system (ROLS reinverted system Volk Medilex, Miami, FL, USA), the Wide Angle Viewing System (WAVS) with the resight non-contact lens (Carl Zeiss Meditec AG, Jena Germany), or recently in the last cases the Zeiss ARTEVO 800 digital ophthalmic three-dimensional (3-D) head-up microscope with the resight non-contact lens system, which was implemented as a hybrid mode (coaxial and 3-D HD 4K monitor), an integrated transoperative OCT allowed retinal structural intraoperative imaging analysis and real-time detection of ERM proliferation, enabling a more precise membrane dissection and stripping. We use diluted triamcinolone acetonide as adjuvant (Kenalog 40 mg/mL; Bristol-Myers Squibb, New York, NY, USA) to better visualize the vitreous adhesions and to safely perform an integral removal of the cortical posterior face from the surface of the retina using a silicone-tipped cannula with active suction. Subsequently, the vitreous base was shaved 360°, assisted with scleral depression. This assisted scleral depression allowed the complete removal of the vitreous traction from the GRT and careful shaving and debulking of the vitreous base using mostly closed port duty cycle with high speed and low vacuum levels to perform a safer shaving of peripheral vitreous mainly over areas of the detached retina without producing iatrogenic retinal tears. Our young patients generally showed vitreous that was attached or only partially detached and removing the core vitreous was relatively straightforward. However, separation of the posterior hyaloid and other areas of adherent vitreous in the periphery with a very mobile retina was technically intricate, especially when concurrent lattice degeneration was present. Injection of a PFCL was used to flatten and unfold the posterior retina. Once the retina was reattached performing meticulous peripheral vitrectomy and ensuring a complete vitreous release with trimming of the anterior giant retinal flap, continuous argon laser endophotocoagulation in three to four rows, mainly at the peripheral edge of the circumferential retinal giant tear and lateral posterior radial extensions (horns tears) of the giant lesion was thoroughly performed.
Additional benefits of the vitrectomy technique in these eyes were the removal of all vitreous opacities, attending to opacified lens capsules, and addressing the cases where significant macular ERM proliferation transoperatively was confirmed. After macular staining using 0.15 mL of a 0.25 mg/mL (0.025%) diluted isomolar solution (pH 7.4) of Brilliant Blue G dye (BBG) we used a 23-gauge diamond-dusted membrane scraper, or 23-, 25-gauge 0.44 ILM forceps (Grieshaber Revolution DSP ILM forceps; Alcon Labs, Fort Worth, TX, USA), and a 23-, 25-gauge Finesse ILM flex loop microinstrument (Grieshaber; Alcon Labs) for the ERM/ILM en-bloc removal on the last surgical cases. In cases where the removal was performed in two steps (double staining technique), trypan blue 0.15% ophthalmic solution (Membrane Blue; Dutch Ophthalmic, Exeter, NH, USA) was instilled under air to remove the ERM proliferations after washing the dye; in the second step, the ILM was stained with the aforementioned BBG dye and removed.
We performed subretinal fluid (SRF) endodrainage very slowly by implementing a first air-fluid exchange over the edge of the GRT to avoid posterior retinal slippage and to remove viscous proteinaceous SRF and to reduce the extent of SRF and to minimize the chance of trapped SRF. To completely dry out the subretinal space, a second air-fluid exchange was performed, a non-expandable bubble containing 15% perfluoropropane (C3F8) gas mixture or lighter than water silicon oil was used as a long-acting tamponade at the end of the procedure in all cases. Figure 2(a-l) shows different surgical approaches.
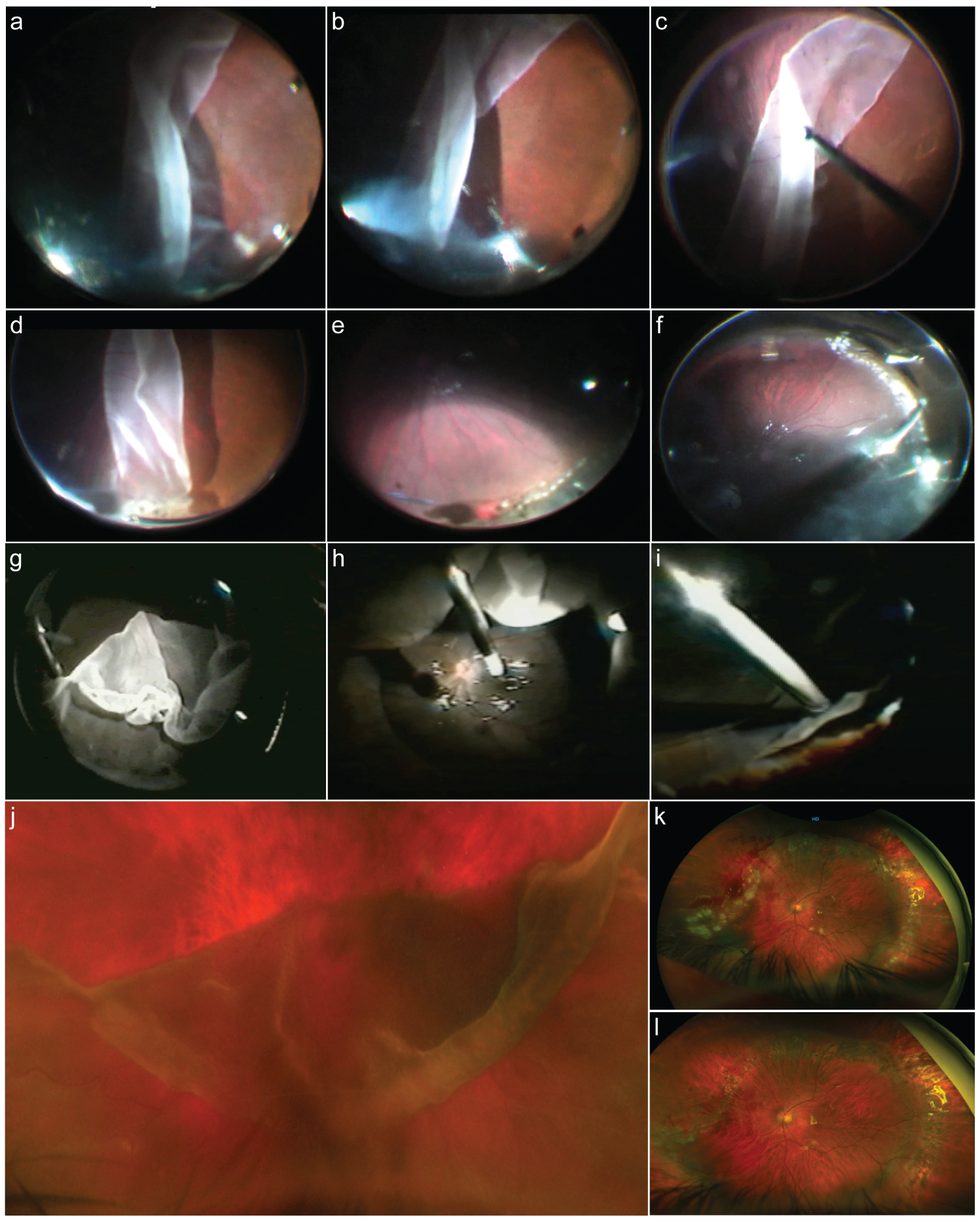 Figure 2: Surgical approaches.
Figure 2: Surgical approaches.
a: Surgical image sequence of giant retinal tear (GRT) anterior vitrectomy in a case with GRT-associated rhegmatogenous retinal detachment (RRD) and posterior retina flap folded over posteriorly. b: Image shows a free posterior flap of the retina once the vitreous has been released. c: Unfolding of the posterior retina flap with careful manipulation. d: Careful anterior vitrectomy at the level of GRT lateral horn. e: Endophotocoagulation of the lateral horn and posterior GRT edge once the retina has been attached assisted with perfluorocarbon liquids. f: Careful fluid-gas exchange with slow endodrainage at the superior border of the GRT. g: Folded and inverted posterior edge of a superior > 180° GRT associated RRD. h: Manipulation and unfolding of the retina at the time of assisted perfluorocarbon liquids injection. i: Trimming of the anterior retina flap along with anterior anomalous condensed vitreous. j: Folded over posterior retinal flap of > 180° superior GRT; there is an inversion of the posterior edge and lateral posterior extension of the lateral horn tear. k: Early postoperative eye that has undergone primary vitrectomy complimented with a high-profile supplement scleral buckle and silicone oil tamponade. l: The previous case 4 months after silicon removal.
View Figure 2
As part of the standardized selected technique of the author, and once the retina is fully reattached only with vitrectomy techniques and laser retinopexy to avoid posterior retinal slippage or radial folds, a methodical, complementary low-lying SB surgical procedure was performed in the eyes with GRTs < 180° consistent with traditional 503, 360° round Lincoff episcleral sponge (Storz model E-5395-4) or standard 240 circling silicon band (style 240/S-2987 by DORC) and 41 circling silicon band (style 41/S-2970 by DORC), the SB was fixed with polyester 5-0 MERSILENE® Polyester Sutures, double-armed 3/8 circle spatulated needle suture (ETHICON, Johnson & Johnson, Brunswick, NJ, USA).
The treatment outcomes included: 1) Long-term postoperative structural SD-OCT findings [Central Sub-Foveal Thickness (CSFT), foveal contour profile, central sub-foveal ellipsoid zone (EZ) status, and central sub-foveal external limiting membrane (ELM) line appearance], 2) En-face imaging or cross-sectional SD-OCT B scan analysis for the presence of dissociated optic nerve fiber layer (DONFL) defects, and 3) The presence of ERM proliferation over the macula.
Statistical tests were selected based on normality of data and the following tests were used: paired Sample t-test (comparison of preoperative and postoperative BCVA), Chi-square test (comparison of categorical data), or repeated ANOVA (before-and-after differences among the tear magnitude groups associated with other study factors). A linear regression analysis was conducted to assess whether CSFT, DONFL defects, ELM line, tear magnitude, and EZ status (inner segment/outer segment band-IS/OS zone) significantly predicted final postoperative BCVA. A multivariate binary logistic regression analysis was performed to evaluate possible factors important for lower final postoperative BCVA. The Kaplan–Meier method evaluated the general survival for final postoperative BCVA between the study groups. All statistical analyses were performed using SPSS 27 (IBM Corp., Armonk, NY, USA), and statistical significance was set at < 0.05.
The clinical charts of consecutive patients diagnosed with GRT-associated RRD who were surgically managed between January 2010 and January 2021 were analyzed. Only eyes with the retina attached and functional vision on the patients' last postoperative evaluations, regardless of the number of surgical procedures needed, were included. Thus, seventy-six eyes from 66 patients were included in the study. Different study groups and the sample size is reported in Table 1, and the demographic data is shown in Table 2.
Table 1: Study groups and sample size. View Table 1
Table 2: Patient's demographic data. View Table 2
The general prevalence of preoperative primary ERM proliferation was 14.4% (11 eyes), but only eight (10.5%) with attached retina underwent ERM/ILM surgery. Additional surgical procedures were required in nine eyes (11.8%) in which significant and symptomatic macular folds were detected due to posterior retinal slippage, vitrectomy review by retained PFCLs in five eyes (6.6%) and reoperation for a macular hole that was not detected in the first surgical time in one eye (1.3%). Different found complications are depicted on Figure 3(a-l). There was no significant difference in postoperative ERM proliferation incidence by tear magnitude (p > 0.05). In total, 43 patients (65.1%) with GRTs of idiopathic etiology and 23 patients (34.8%) who were considered to have GRTs associated with high myopia, connective tissue alterations (Marfan-Stickler syndrome), and severe closed-eye contusion trauma were included (Table 3).
Table 3: Clinical and surgical data of the operated patients. View Table 3
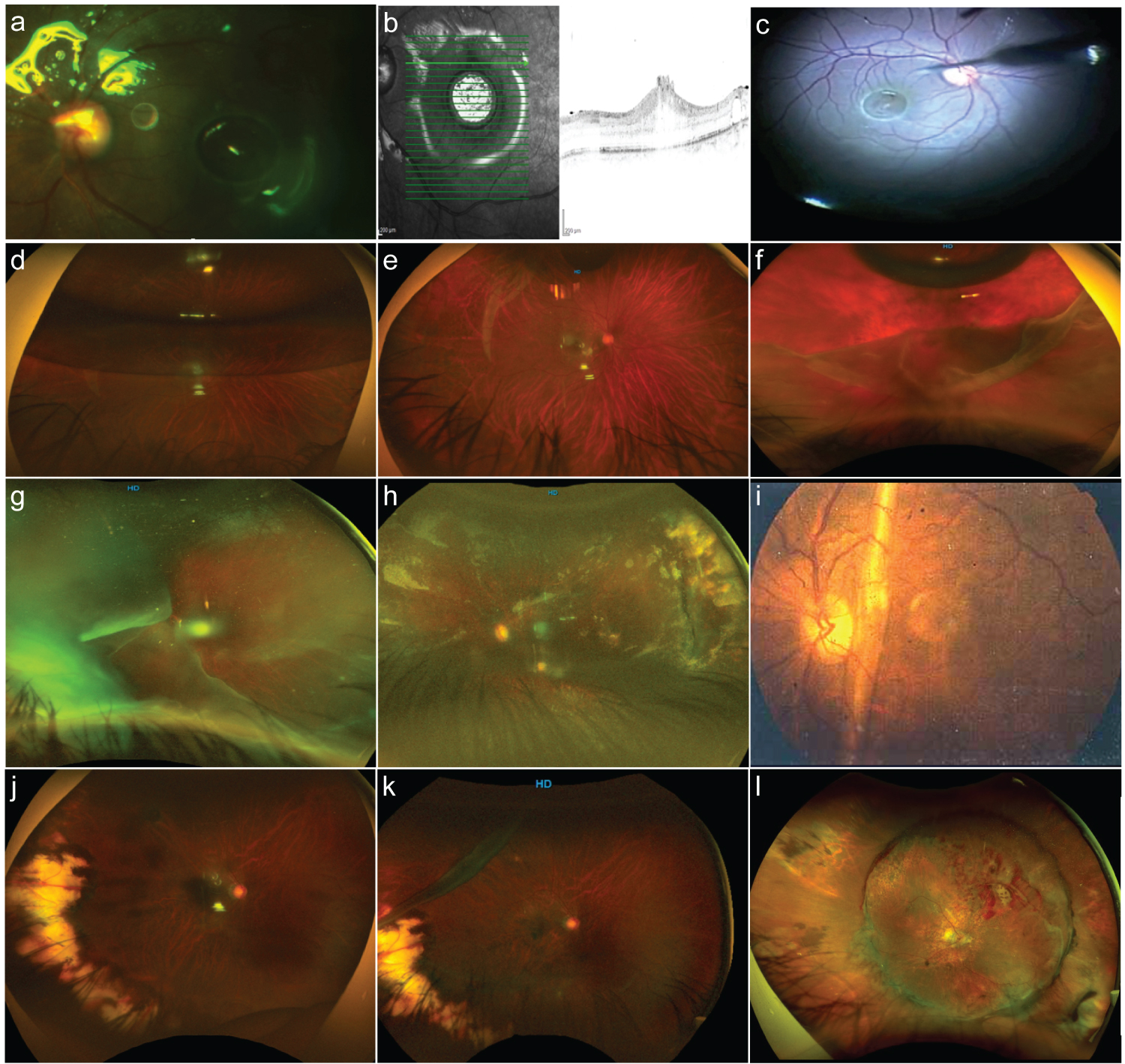 Figure 3: Giant retinal tears complications.
Figure 3: Giant retinal tears complications.
a: Discrete amount of subretinal perfluorocarbon liquid at the level of the macula. b: Corresponding spectral-domain optical coherence tomography (SD-OCT) B scan image depicting the presence of retinal macula edema and defects around due to the presence of perfluorocarbon liquid. c: The surgical intraocular 41-G needle pointed to the subretinal perfluorocarbon liquid bubble extraction. d: Postoperative 40% gas-filled eye with some posterior retinal flap slippery. e: A light posterior slippage of the posterior retina; however, there is a remarkable retinal fold of the superior edge of the giant retinal tear (GRT), and the retina remains attached. f: A 10% gas-filled eye with a reopened GRT-associated rhegmatogenous retinal detachment (RRD) and some degree of PVR. g: A temporal GRT with the detached retina folded over the optic nerve. h: The post operated eye of the previous image with cloudy media and evidence of attached retina. i: Very old collection image of an eye belonging to this set of patients with GRT showing a macular vertical full-thickness vertical fold after primary vitrectomy with a complimented scleral buckle. j: The postoperative eye 8 months after vitrectomy and scleral buckle for temporal side macula-on GRT. k: The same patient in the previous image having a recurrent RRD due to a new GRT superior to the previous lesion and having a previous vitrectomy with a scleral buckle. l: A case in the early postoperative period depicting evidence of a totally attached retina, high scleral buckle, and evidence of bleeding along the posterior edge of the giant retinal tear.
View Figure 3
The statistical program yielded the following SD-OCT abnormalities in the macula-off GRT-associated RRD group: EZ disruption was observed in 57.9%, CSFT abnormalities in 74.7%, and ELM line alteration in 42.1% of the eyes. In the macula-on GRT-associated RRD group, EZ disruption was observed in 41.3%, CSFT abnormalities in 62.3%, and ELM line alteration in 51.9% of eyes (Figure 4a-j and Figure 5a-l). The differences between these categorical variables were not significant (p > 0.05%).
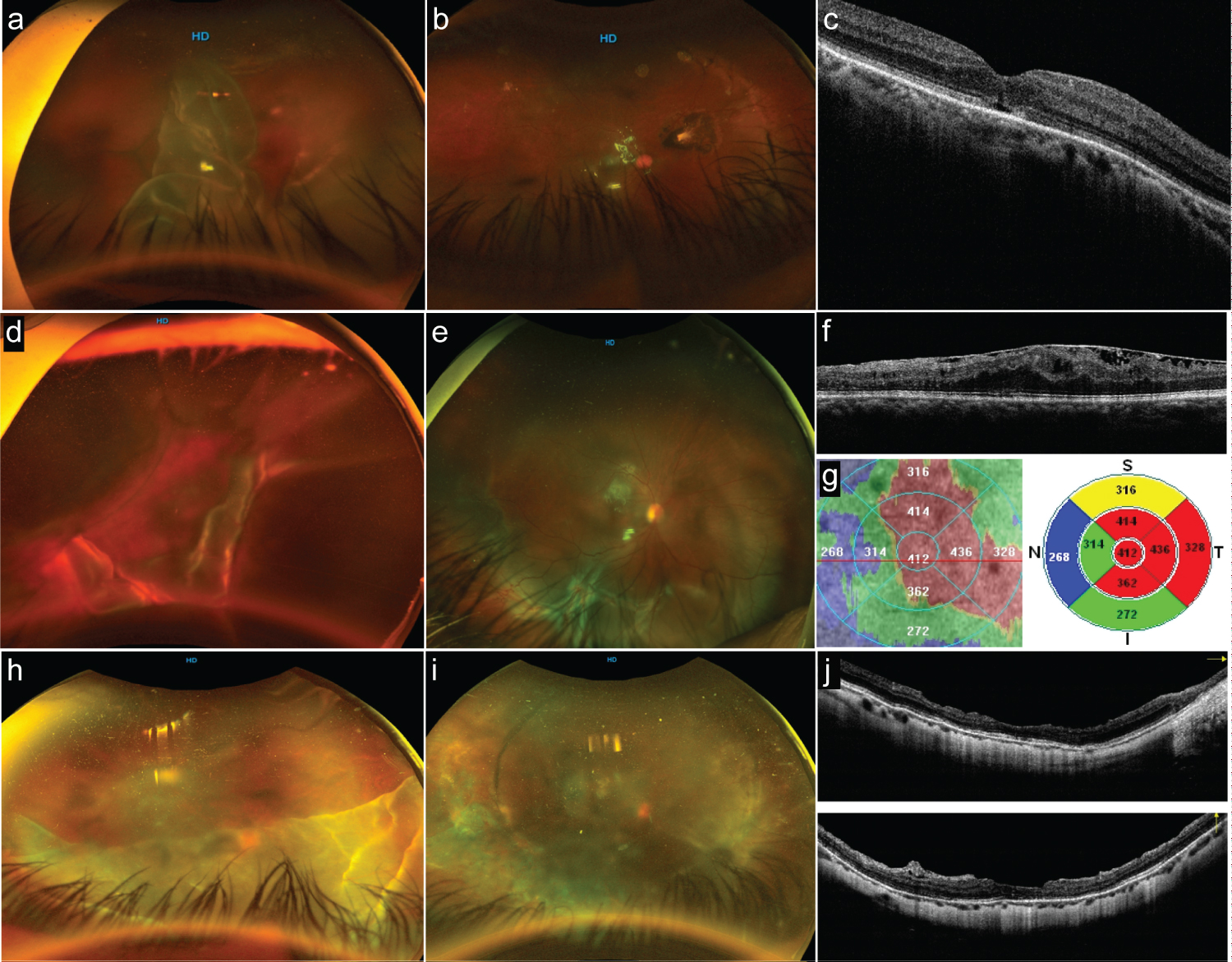 Figure 4: Postoperative optical coherence tomography (OCT) (part 1).
Figure 4: Postoperative optical coherence tomography (OCT) (part 1).
a: Preoperative status of an eye with giant retinal tear (GRT) from > 180° in extension showing a complete posterior vitreous detachment and rolled-back posterior retina flap. b: The postoperative status with a totally reattached retina and without evidence of macular epiretinal membrane (ERM) proliferation. c: The long-term postoperative horizontal B-scan spectral-domain (SD)-OCT image; the foveal contour is normal, central subfoveal thickness is within normal range, and the internal segment/external segment (IS/OS) (ellipsoid band) and external limiting membrane (ELM) line show subfoveal reflectance discontinuities. d: Optos image showing a complex GRT with opaque media; there is superior lateral posterior retinal tear extension, and the posterior retina looks folded over. e: The postoperative status of the eye; the eye has undergone primary vitrectomy and gas tamponade. The inferior retina exhibits a full-thickness retinal fold due to posterior retina slippage and evidence of radial fold due to posterior PVR. f: Macular expression of extended posterior PVR to the macula in the form of ERM proliferation; this corresponding SD-OCT horizontal B-scan shows wrinkling of the superficial layers of the macula, the external layers are difficult to identify, the IO/OS band and ELM show discontinuities, and some amount of residual subretinal fluid is seen. g: Composite image showing a topographic irregular thickening of the macula. h: Optos image of a superior GRT > 180° folded over itself, obscuring the macula status. i: The postoperative status of the eye with a 360° buckle and silicone oil as tamponade with evidence of irregular but confluent 360° endolaser retinopexy. j: A crossline SD-OCT through the center of the fovea once the silicon has been removed. The horizontal and vertical B-scan show an irregular foveal contour, with some diffuse irregular retinal thinning, some posterior vitreous cortical remnants, and some extrafoveal dissociated optic nerve fiber layer defects.
View Figure 4
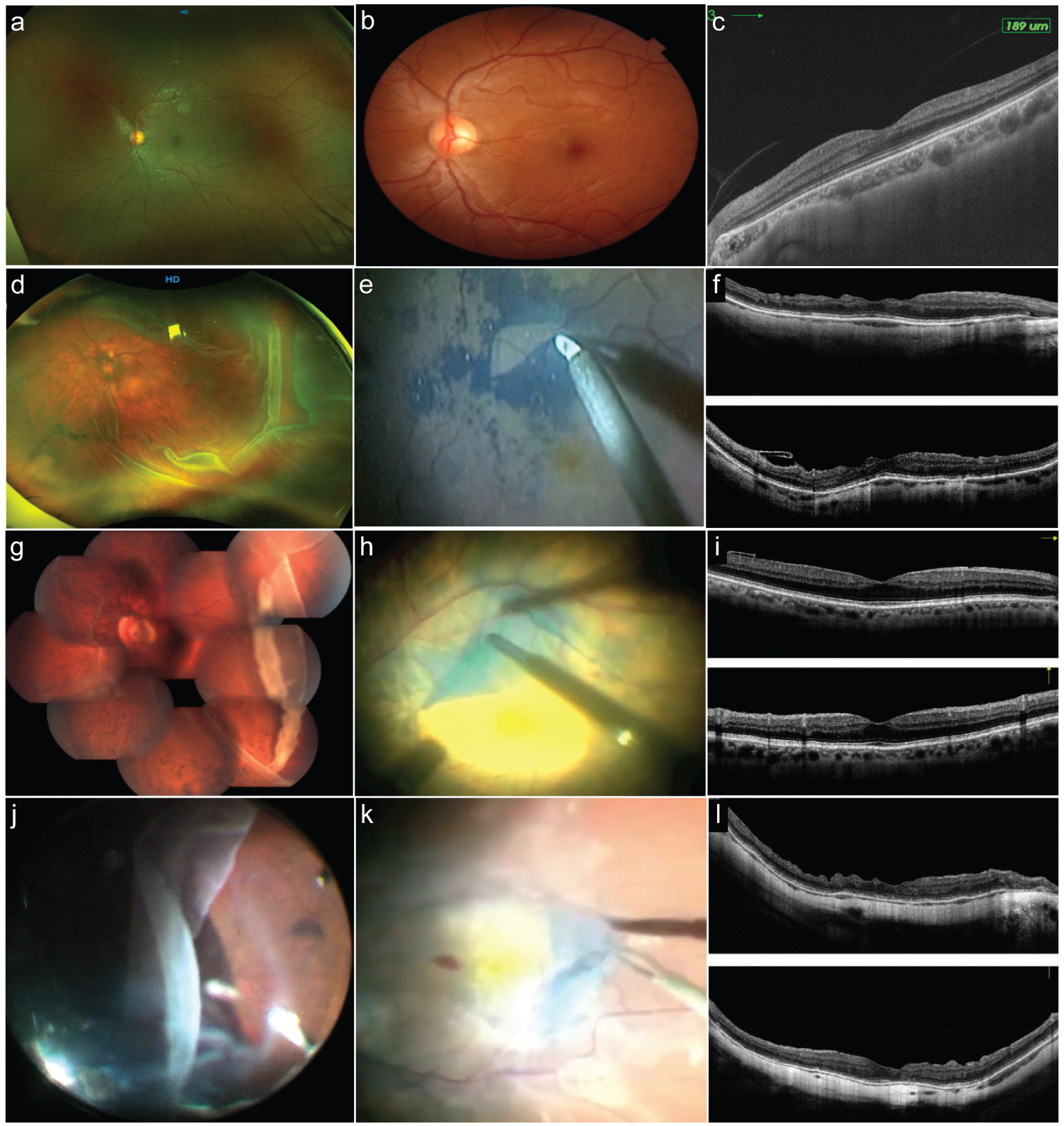 Figure 5: Postoperative optical coherence tomography (OCT) (part 2).
Figure 5: Postoperative optical coherence tomography (OCT) (part 2).
a: Control normal wide-field Optos fundus photo. b: Magnified detail of the normal 30° wide color fundus. c: Spectralis normal control spectral-domain optical coherence tomography (SD-OCT) through the center of the fovea B horizontal scan. d: Temporal giant retinal tear (GRT) case with macula on rhegmatogenous retinal detachment (RRD) that has developed epiretinal membrane (ERM) proliferation after the surgical procedure. e: Surgical view of the Brilliant Blue G-stained internal limiting membrane removal technique. f: The previous case showing crossline SD-OCT horizontal and vertical B scans depicting abnormal foveal contour and dissociated optic nerve fiver layers (DONFL) defects; Henle fiber layer and outer nuclear layer are abnormal; the external limiting membrane line (ELM) and the inner segment/outer segment (ellipsoid band) look preserved. g: Image of composite > 180° GRT figure with some subretinal blood over the posterior pole, the eye has undergone uneventful primary vitrectomy and endolaser retinopexy; and after 8 weeks it has developed a secondary epiretinal membrane proliferation over the macula. h: ERM and internal limiting membrane (ILM) en-bloc membrane removal technique. i: Postoperative crossline SD-OCT 14 months after ERM removal; the horizontal B scan shows a normal foveal profile, with ILM remnants temporal to the center of the fovea. j: The same clinical case as the corresponding in Figure 1a, where the traction vitreous releases from the anterior retinal flap of a GRT. k: Stained ERM of the previous case. l: Crossline SD-OCT horizontal and vertical through the center of the fovea of the previous case where the normal foveal profile is lost; there are both horizontal and vertical scan DONFL defects and some discontinuities on the ELM line and the ellipsoid band.
View Figure 5
The postoperative visual acuity (0.56 ± 0.26 logMAR) was significantly better than the preoperative visual acuity (1.07 ± 0.61 logMAR) (p < 0.001) (Table 1). The Spearman's rank correlation coefficient test showed a moderate negative correlation (rho = −0.53, p < 0.01) of the postoperative BCVA (logMAR) with the CSFT in microns (Figure 6).
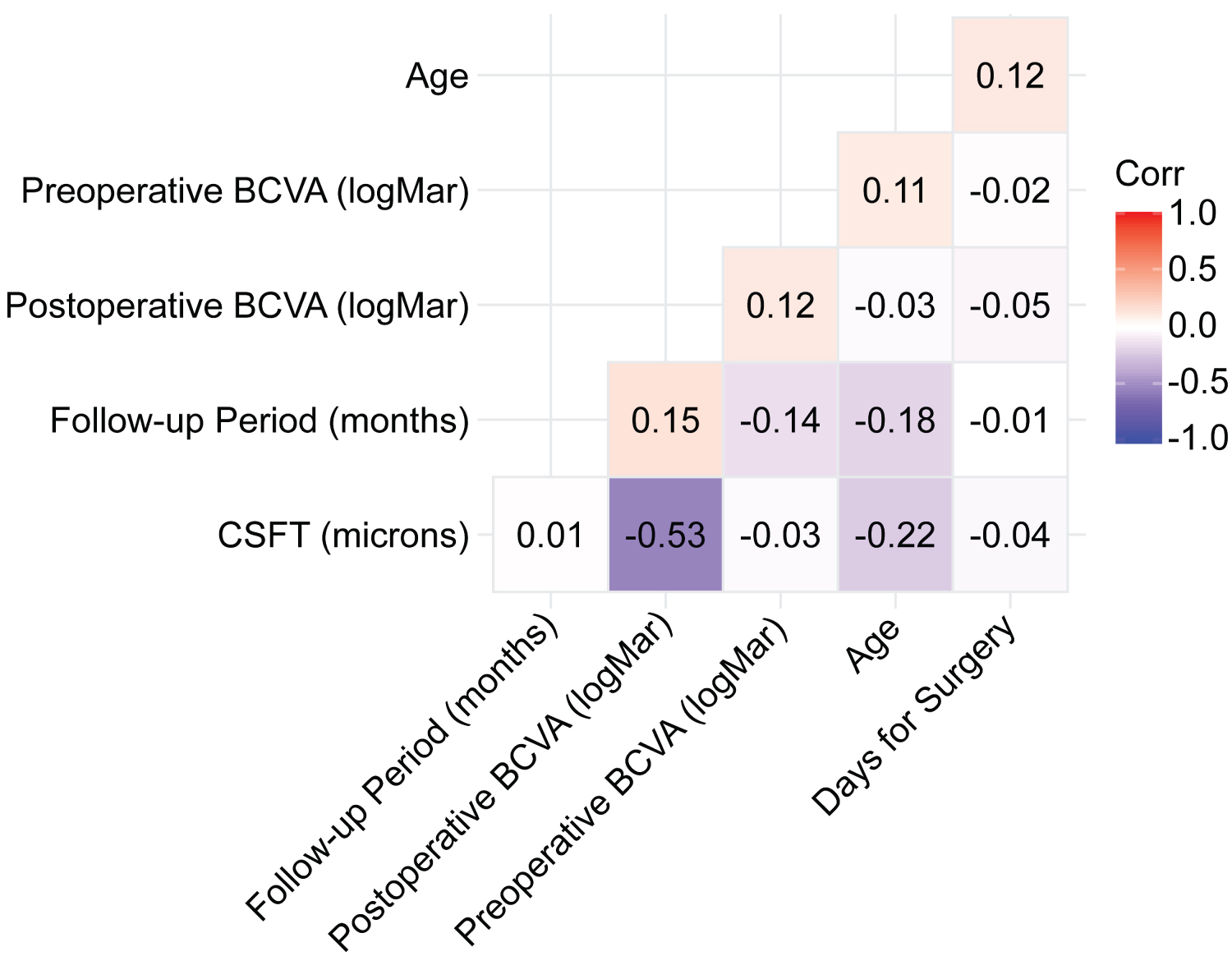 Figure 6: The Spearman's rank correlation coefficient. The Spearman's rank correlation coefficient test shows a moderate negative correlation (rho = -0.53; p < 0.01) of the postoperative best-corrected visual acuity (BCVA) (logMAR) with the central subfoveal thickness in microns.
View Figure 6
Figure 6: The Spearman's rank correlation coefficient. The Spearman's rank correlation coefficient test shows a moderate negative correlation (rho = -0.53; p < 0.01) of the postoperative best-corrected visual acuity (BCVA) (logMAR) with the central subfoveal thickness in microns.
View Figure 6
Results suggested a statistical relationship between tear magnitude and etiology (p < 0.001). There were differences in final postoperative BCVA in logMAR units among the levels of tear magnitude, although the data were not statistically significant (P > 0.05) (Table 3). The eta squared was 0.16, implying that the tear magnitude explained approximately 16% of the variance in final postoperative BCVA. Regarding the main effect of tear magnitude, the mean final postoperative BCVA for tear magnitude was significantly larger than for tear > 270° (p = 0.048) (Figure 7). For the main effect of tear magnitude, the mean of final postoperative BCVA for tear 180-270° was significantly larger than for tear > 270° (p = 0.001) (Figures 8). No other significant effects were found. There was also a significant relationship between tear magnitude and tamponade type based on an alpha value of 0.05, χ2(2) = 7.69, p = 0.21.
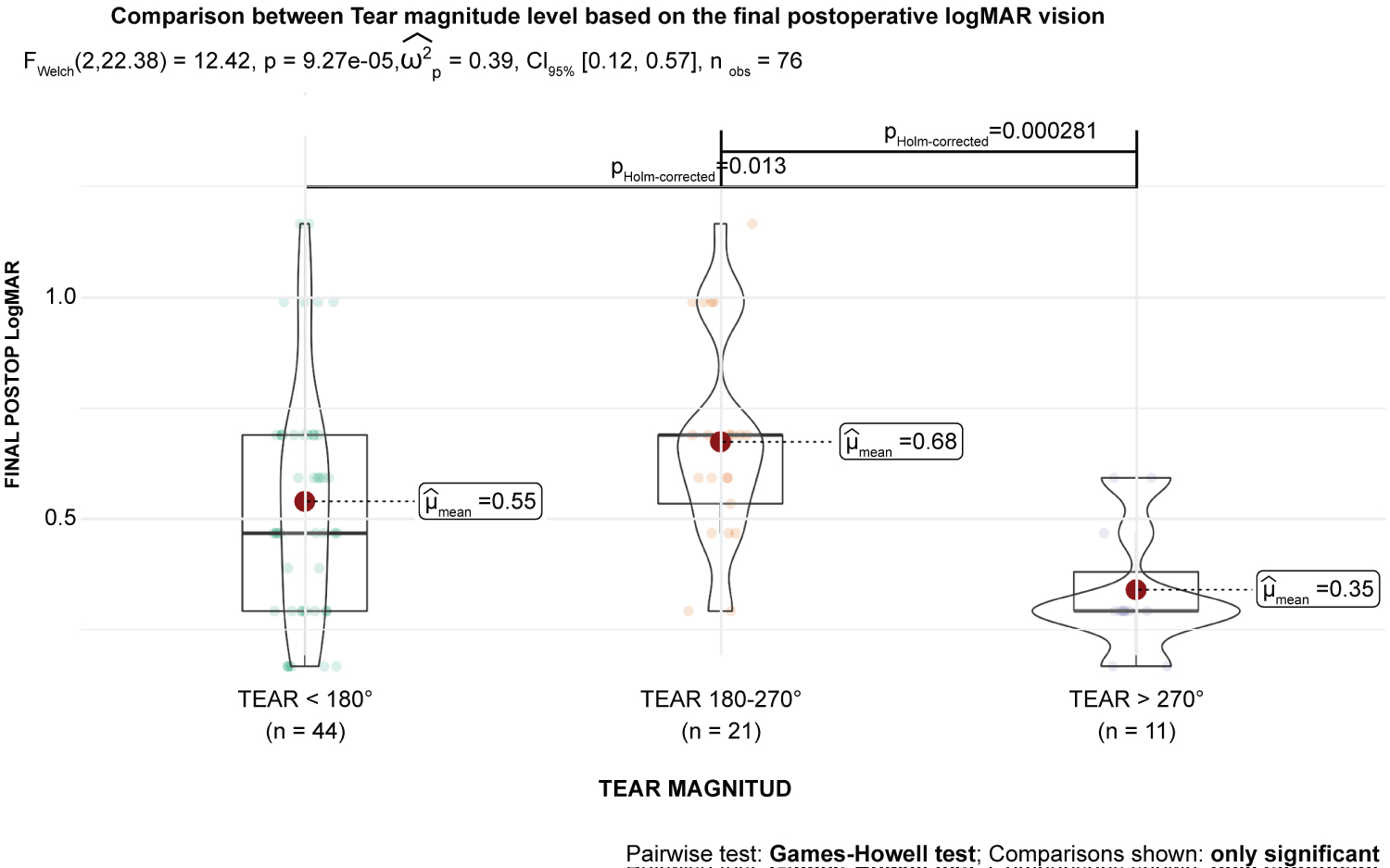 Figure 7: Tear magnitude level based on the final postoperative best-corrected visual acuity (BCVA) in logMAR units. This figure suggests that the tear 180-270° category of tear magnitude does not significantly predict final postoperative BCVA in logMAR units (B = 0.09; t [69] = 1.57; p = 0.120). Based on this sample, this suggests that moving from the tear 270° category of tear magnitude does not significantly predict final postoperative BCVA in logMAR units (B = -0.11; t [69] = -1.43; p = 0.157) or have a significant effect on the mean of final postoperative BCVA in logMAR units.
View Figure 7
Figure 7: Tear magnitude level based on the final postoperative best-corrected visual acuity (BCVA) in logMAR units. This figure suggests that the tear 180-270° category of tear magnitude does not significantly predict final postoperative BCVA in logMAR units (B = 0.09; t [69] = 1.57; p = 0.120). Based on this sample, this suggests that moving from the tear 270° category of tear magnitude does not significantly predict final postoperative BCVA in logMAR units (B = -0.11; t [69] = -1.43; p = 0.157) or have a significant effect on the mean of final postoperative BCVA in logMAR units.
View Figure 7
High Variance inflation factors (VIFs) indicate increased effects of multicollinearity in the model. VIFs > 5 are cause for concern, whereas VIFs of 10 should be considered the maximum upper limit [15]. VIFs for all predictors (CSFT, DONFL defects, ELM line, Tear magnitude, and EZ (IS/OS zone) in the regression model were < 10.
The results of the linear regression model were significant (p < 0.001), indicating that approximately 37% of the variance in final postoperative BCVA in logMAR units were explained by CSFT in microns, DONFL defects, ELM line, tear magnitude, and EZ status (IS/OS zone) (Table 4) [16]. CSFT significantly predicted the final postoperative BCVA (p < 0.001). This indicated that, on average, a one-unit increase of CSFT in microns decreased the value of final postoperative BCVA in logMAR by 0.00 units.
Table 4: Regression model parameter estimates to measure the association of the OCT biomarkers. View Table 4
There was a significant difference between the values of preoperative BCVA and final postoperative BCVA after controlling for tear magnitude and tamponade type (p < 0.001). Also, the relationship between preoperative BCVA and final postoperative BCVA differed significantly between the levels of tear magnitude (p < 0.001) (Figure 9). Tamponade type was significantly related to preoperative BCVA and final postoperative BCVA (p = 0.022). Binary logistic regression analysis showed that the overall model was significant (p < 0.001), suggesting that months of follow-up, tear magnitude, CSFT in microns, status of the macula at surgery, recurrent RRD, postoperative foveal contour appearance, EZ status, DONFL defects, and ELM line appearance had a significant effect on the odds of observing the 20/300 or worse category of postoperative BCVA.
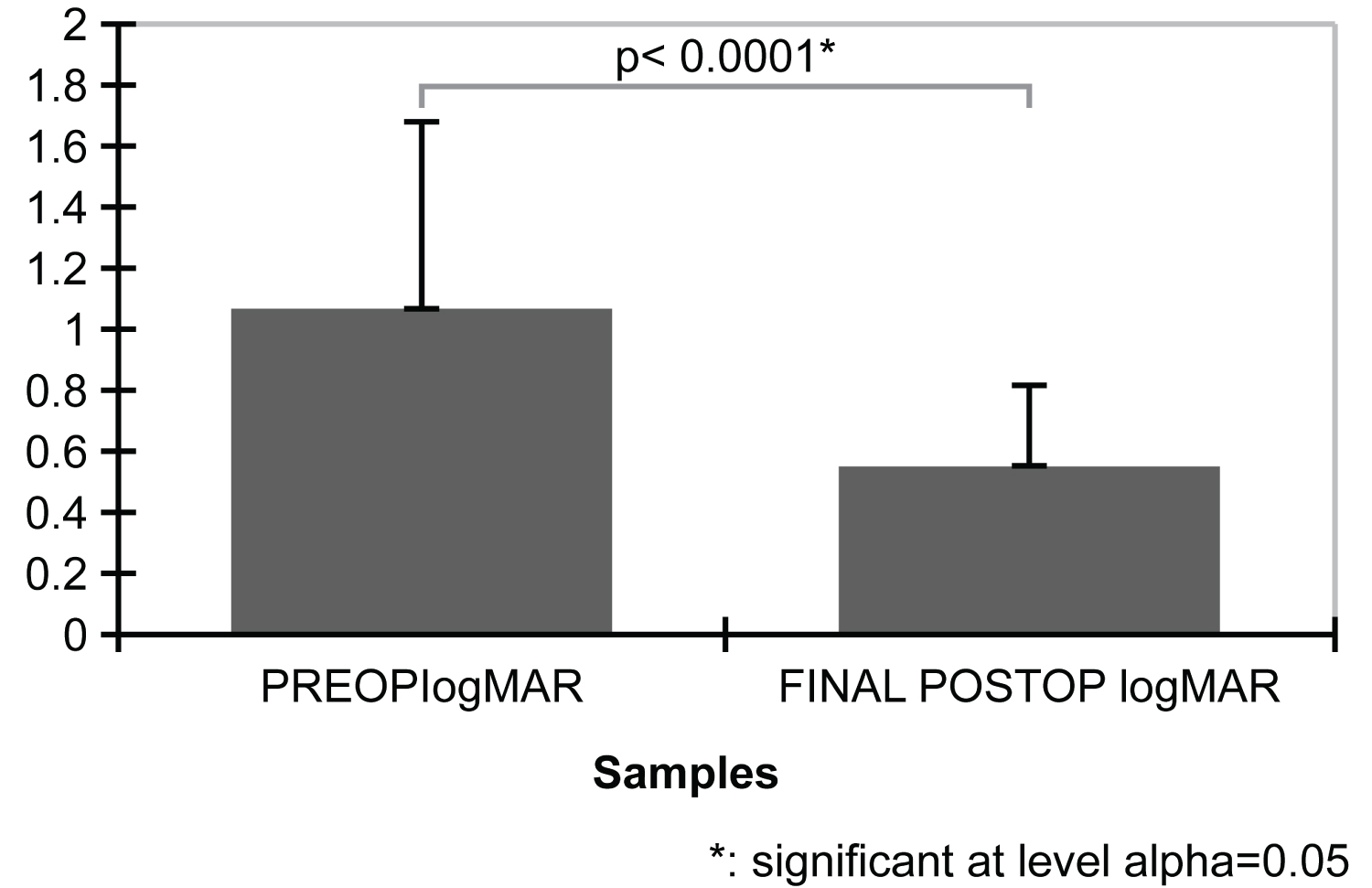 Figure 8: Bar plots mean preoperative best-corrected visual acuity (BCVA) and final postoperative BCVA in logMAR units. The result of the two-tailed paired samples t-test is significant based on an alpha value of 0.05 (t [75] = 6.88; p < 0.001), indicating that the null hypothesis can be rejected. Thus, the difference in the mean preoperative BCVA in logMAR units and the mean of final postoperative BCVA in logMAR units is significantly different from zero. The mean preoperative logMAR is significantly higher than the mean final postoperative BCVA in logMAR units.
View Figure 8
Figure 8: Bar plots mean preoperative best-corrected visual acuity (BCVA) and final postoperative BCVA in logMAR units. The result of the two-tailed paired samples t-test is significant based on an alpha value of 0.05 (t [75] = 6.88; p < 0.001), indicating that the null hypothesis can be rejected. Thus, the difference in the mean preoperative BCVA in logMAR units and the mean of final postoperative BCVA in logMAR units is significantly different from zero. The mean preoperative logMAR is significantly higher than the mean final postoperative BCVA in logMAR units.
View Figure 8
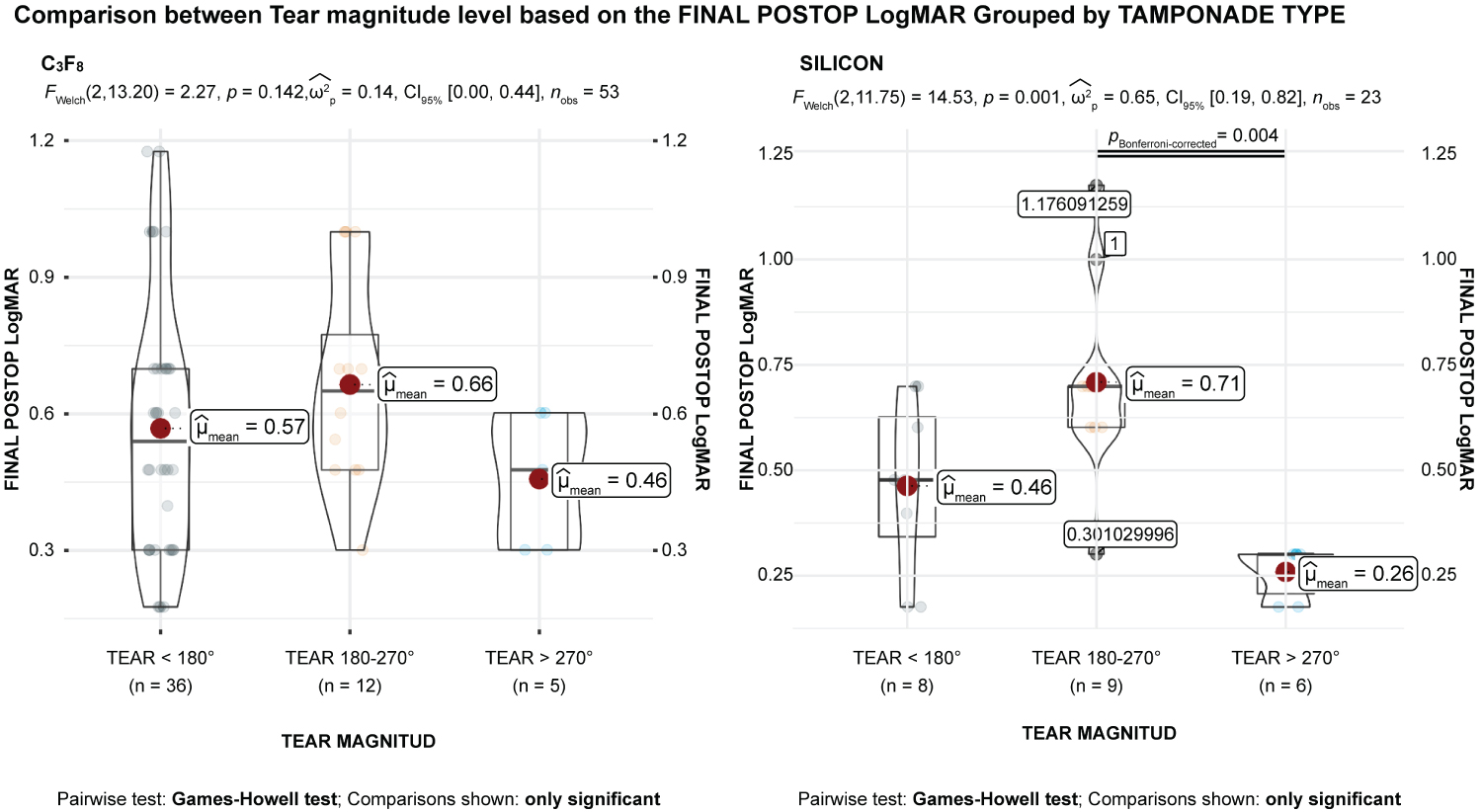 Figure 9: The means comparisons between tear magnitude and tamponade type. Tamponade type is significantly related to preoperative best-corrected visual acuity (BCVA) and final postoperative BCVA in logMAR units (F [2,71] = 4.01; p = .022). The main effect for the within-subjects factor is significant (F [1,71] = 39.19; p < 0.001), indicating significant differences between the values of preoperative BCVA and final postoperative BCVA in logMAR units after controlling for tear magnitude and tamponade type. The interaction effect between the within-subjects factor and tear magnitude is significant (F [2,71] = 8.66; p < 0.001), indicating that the relationship between preoperative BCVA and final postoperative BCVA in logMAR units differs significantly between the levels of tear magnitude. The interaction effect between the within-subjects factor and tamponade type is not significant (F [2,71] = 1.71; p = 0.188), indicating that the relationship between preoperative BCVA and final postoperative BCVA in logMAR units is similar between the levels of tamponade type.
View Figure 9
Figure 9: The means comparisons between tear magnitude and tamponade type. Tamponade type is significantly related to preoperative best-corrected visual acuity (BCVA) and final postoperative BCVA in logMAR units (F [2,71] = 4.01; p = .022). The main effect for the within-subjects factor is significant (F [1,71] = 39.19; p < 0.001), indicating significant differences between the values of preoperative BCVA and final postoperative BCVA in logMAR units after controlling for tear magnitude and tamponade type. The interaction effect between the within-subjects factor and tear magnitude is significant (F [2,71] = 8.66; p < 0.001), indicating that the relationship between preoperative BCVA and final postoperative BCVA in logMAR units differs significantly between the levels of tear magnitude. The interaction effect between the within-subjects factor and tamponade type is not significant (F [2,71] = 1.71; p = 0.188), indicating that the relationship between preoperative BCVA and final postoperative BCVA in logMAR units is similar between the levels of tamponade type.
View Figure 9
The regression coefficient for months of follow-up was significant (p = 0.020), indicating that for a one-unit increase in months of follow-up, the odds of observing the 20/300 or worse category of postoperative BCVA increased by approximately 4%. Additionally, the regression coefficient for macula-off at surgery was significant (p = 0.04), indicating that for a one-unit increase in macula-off at surgery, the odds of observing the 20/300 or worse category of postoperative BCVA increased by approximately 638%. However, the regression coefficient for foveal contour appearance was significant (p < 0.001), indicating that for a one-unit increase in foveal contour abnormality, the odds of observing the 20/300 or worst category of postoperative BCVA also increased.
A Cox proportional hazards model was used to determine whether tear magnitude significantly affected the hazard of final postoperative BCVA categories. The 20/300 or worse category of final postoperative BCVA was used to indicate survival, while the 20/50 to 20/200 group category was used to represent a hazard event. The model's results were significant (p = 0.006), indicating that tear magnitude adequately predicted the hazard of the final postoperative BCVA. The coefficient for the tear 180-270° group category of tear magnitude was significant (p = 0.027), indicating that at any particular time, an observation in the tear 180-270° category had a hazard that is 0.33 times larger on the final postoperative BCVA. Kaplan-Meier survival probability plot was included for tear magnitude. Each plot represents the survival probabilities of the different groups over time (Figure 10). A calculation of survival probability according to the tear magnitude was performed, and the results are shown in risk tables (Table 5 and Table 6).
Table 5: Risk table for tear magnitude = tear 180-270° View Table 5
Table 6: Risk table for tear magnitude = tear > 270°. View Table 6
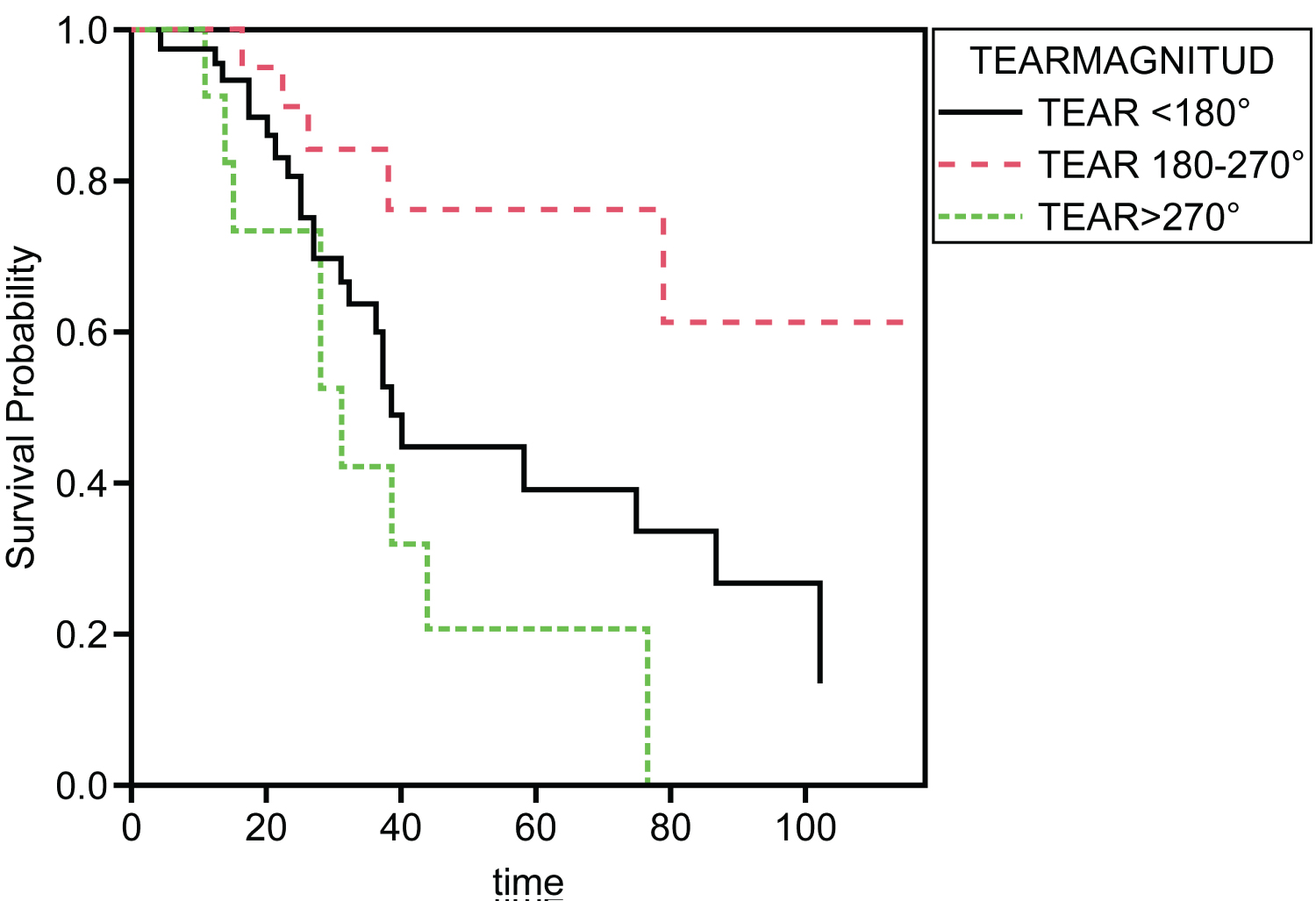 Figure 10: Kaplan-Meier survival plots. A Kaplan-Meier survival probability plot is included for tear magnitude. Each plot represents the survival probabilities of the different groups over time.
View Figure 10
Figure 10: Kaplan-Meier survival plots. A Kaplan-Meier survival probability plot is included for tear magnitude. Each plot represents the survival probabilities of the different groups over time.
View Figure 10
Additionally, we used generalized linear models (GLM) to further investigate potential associations of the postoperative BCVA with the other variables. The selected by "step" generalized additional model for the postoperative BCVA revealed that the postoperative BCVA was significantly associated with the number of follow up months (p < 0.01), the CSFT (p < 0.01), and the "Normal foveal contour" (p < 0.01), when adjusting for cofounders with multivariate analyses (Table 7).
Table 7: The "best" generalized linear model results of the postoperative BCVA in logMAR units. View Table 7
The present study's data showed no difference in the degrees of tear magnitude between patients' age or sex. However, there was an influence of the cause that led to GRTs-associated RRD. Most cases of tears < 180° and 180° to 270° were idiopathic, while most cases of tears > 270° were related to Marfan or Stickler-Wagner Syndrome. It is important to know that, in addition to disease-related causes, the idiopathic etiology is of concern and an early and accurate diagnosis of ruptures is essential [17].
Findings on the benefit of adding an encircling SB to vitrectomy for GRTs-associated RRD are controversial. Studies that favor buckling argue that GRTs occur in patients with an abnormal vitreous base and that supporting this region decreases the tractional forces that could reopen the break or cause a tear extension into the uninvolved retina [18,19]. Although it can facilitate the removal of the anterior vitreous when placed before reattaching the retina, it favors posterior slippage of the retina. It is why we always place the scleral buckle after full transoperative reattachment of the retina. Other authors believe that buckling is an essential part of the surgical approach, as it contributes to relieving the traction at the edges of the GRT and provides support for the rest of the vitreous base, mainly at its posterior border [20].
Some degree of general agreement among retinal surgeons has led to the idea of using scleral buckling in GRTs < 180°, especially in the absence of total posterior vitreous detachment (PVD) and folding of the retina on itself. However, some authors argue that GRTs, especially if their extent is > 180°, already involve a significant release of vitreous traction. Consequently, it is unnecessary to adopt an SB if much of the vitreous traction has already been released. Also, it is irrelevant if the goal is merely to ensure that the posterior retina is released and free of possible abnormal vitreoretinal adhesions commonly seen in this condition. In these cases, trimming off the devitalized anterior retina is advised to minimize the occurrence of anterior PVR or abnormal neovascularization [19].
Our surgical technique helped minimize the chance of posterior retinal slippage and reduced the chance of producing a posterior radial retinal fold at the time of fluid-gas exchange. These are known to increase the risk of leakage from tears and increase the recurrence rate for RRD. In complex PVR total RRD cases, when we decided to use a higher-lying buckle, the goal was to position the posterior edge of the GRT to fall on the plateau of the indentation and to release and avoid pathological contraction of the residual vitreous base over the detached but not torn retina.
Regarding vitreous surgical management in cases associated with trauma (vitreous base avulsion) or genetic syndromes (Marfan syndrome, Stickler-Jansen syndrome, etc.), PVD was less commonly present, and many abnormal vitreoretinal adhesions were found. Consequently, thorough, full dissection of the vitreous base is advised, with the aid of scleral depression, if available, as was done in our study. In most cases, we performed assisted scleral depression or on the last surgical cases self-scleral depression assisted with a single 27-gauge disposable Eckard TwinLight Chandelier, as described previously [21]. Present results suggest that early surgery prevents future damage to the eye, which was evident in survival analysis of different tear magnitudes where patients with a tear magnitude of 180°-270° had the best probability of survival while patients with a tear magnitude > 270° had the worst survival rate.
Lensectomy or clear lens removal is unnecessary for most cases unless lenticular opacity is an obstacle [22]. In this study we observed that giant retinal tears of smaller magnitude are those that require a more extensive shaving of the vitreous base and consequently lensectomy or phacoemulsification of the lens to achieve it more efficiently, but this variable and its clinical repercussions were not statistically evaluated. Also, there is no scientific evidence to support the addition of a 360° endolaser in the absence of lattice degeneration [19]. Accordingly, we do not regularly apply 360° endolaser, even for GRTs with extents < 180°. Instead, we prefer to support the residual vitreous base with a low-lying buckle, as previously mentioned. Fluid-air exchange is arguably the most critical step in the surgical repair of GRT-associated RRDs, and an improper technique can result in retinal slippage or full-thickness radial retinal folds. Careful, slow aqueous removal by means of air-fluid exchange with an extrusion cannula at the fluid-PFCL interface and at the edge of the tear is essential. Our study showed a very low incidence of macular retinal folds due to posterior slippage in eyes with GRTs extending < 180°, which was higher (but not significantly so) in GRTs extending > 180°.
Concerning the type of tamponade, we avoided the use of short-acting gas (sulfur hexafluoride) because there is evidence that suggests it leads to higher rates of re-detachments [22]. Our data suggested that tear magnitude and tamponade type were related. In this study, we only used 15% nonexpanding, long-acting C3F8 gas or, for very rare cases of poor compliance with postoperative positioning in eyes with primarily inferior GRTs, lighter than water silicone oil. In contrast, Kunikata, et al. [23] used silicone oil as a tamponade in 34 of 41 eyes (83%) of its GRT-associated RRD.
In this study, we found a bilateral GRT incidence of 6.57%. Patients who experience spontaneous GRT-associated RRD are at a high risk of developing GRT in the fellow eye [3]. High-risk fellow eyes include those with high myopia, Wagner or Stickler syndrome, and progressively increasing areas of white-without-pressure (WWOP) with a sharp posterior margin and increased vitreous base condensation [3]. Although neither the annual nor cumulative risk after 3, 5, or 7 years of GRT-associated RRD in the fellow eye has been precisely defined, there is substantial potential visual morbidity associated with GRT-associated RRD, and many authors have discussed the use of lasers and even surgical prophylaxis in the fellow eye [18,19].
The need for prophylaxis remains controversial, and some authors advocate for retinopexy for any peripheral retinal breaks or lattice degeneration in the fellow eye. Patients with a syndromic connective tissue predisposition and certain local ocular, high-risk peripheral retinal lesions such as WWOP or vitreous conditions such as abnormal condensation of the vitreous base are especially at high risk and are deserving of close monitoring or even discussion of the role of a highly debated, thorough, prophylactic buckle [24]. Some authors advocate prophylactic, 360° cryotherapy posterior to the ora serrata in the fellow eye of persons with Stickler syndrome, [24] while others have suggested prophylactic buckling along with cryopexy [3]. Prophylactic 360° laser photocoagulation can also be considered, especially in eyes with multiple risk factors.
In this report, major postoperative complications other than PVR included symptomatic macular folds associated with retinal posterior slippage as reported in other studies [8,25]. If PVR is present, visual prognosis is poor despite reattachment and anatomic success, as demonstrated in the present study and a previous study [1]. In contrast, Kunikata, et al. [23] reported that the most common postoperative complications occurred in 16 eyes (39%) including macular ERM proliferation, cystoid macular edema, macular hole, subretinal perfluorocarbon liquid, retinal folds, vitreous hemorrhage, and re-detachment.
Some authors [26] have emphasized that in non-complex cases, good anatomic and functional results can be obtained only with vitrectomy techniques. We observed a 79% rate of reattachment after one surgical procedure, which is consistent with the 75% single surgery anatomic success at 3 months and 65% at 2 years (95 confidence interval: 47%, 78%), as reported by Li, et al. (2021) [26]. The analysis of the results of a prospective study in the management of different types of retinal detachments concluded that the GRTs-associated RRD is associated with a complex high-risk pathology. Eyes that underwent even timely surgery hardly recovered vision of 20/40 or better [27]. The risk factors for poor visual recovery include old age (> 70 years), low intraocular pressure (< 10 mmHg), retinal detachment with more than four rhegmatogenous lesions, and retinal detachment of more than three quadrants [27].
Multivariate logistic regression analysis was performed to identify the risk factors such as presenting with worse visual acuity, ≥ 150° giant retina tear, macula-off status, and presence of PVR were associated with postoperative BCVA of > 1.0 in logMAR units (all p < 0.05) in the present study. However, the types of surgery (primary vitrectomy vs. combined SB and vitrectomy techniques), number of breaks, lens extraction, and additional cryotherapy were not associated with functional or anatomic success, according to Ting, et al. [28].
The present study has inherent limitations due to its retrospective design, which is not different than other similar studies. There were several strengths, such as its long-term follow-up data availability. Also, the same surgeon performed all surgical procedures, which is known to eliminate the surgeon-bias factor. Additionally, a relative rarity of GRT-associated RRD allowed us to incorporate a structural evaluation by means of long-term final postoperative SD-OCT data to be correlated with final postoperative BCVA.
Our data showed multiple structural alterations in SD-OCT biomarkers. Eyes that developed secondary epiretinal membrane proliferation showed significantly improved BCVA after proliferation, and the ILM was removed. The structural findings correlated with the BCVA. Despite a fully reattached retina without ERM proliferation, GRTs-associated RRD has a guarded functional prognosis, and an early surgical intervention can achieve better visual outcomes.
This retrospective study adhered to the tenets of the Declaration of Helsinki, received full ethical approval from the research ethics committee, and was approved by the institutional review committee and the teaching department of the institution enrolled (no reference numbers were provided for retrospective studies by this institution). Written informed consent was obtained from all patients in accordance with the institutional guidelines.
Written Informed consent was obtained from all participants included in the study.
The authors affirm that participants provided informed consent for the publication of all images in Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5 from selected patients, as well as the images in the online resources, if any.
Dataset supporting the conclusions of this article is included within the article. Dr. Miguel A. Quiroz-Reyes, states that he has no financial disclosures relevant to the content of this article. He may be reached at drquiroz@prodigy.net.mx
Photos and composite figures supporting the findings of this report may be released upon written application to the Photographic Laboratory and Clinical Archives Department of Retina Specialists Unit at Oftalmologia Integral ABC, Medico surgical Assistance Institution (non-profit organization), Paseo de las Palmas 735 suite 303. Bosques de Chapultepec. Alcaldia Miguel Hidalgo. Mexico City 11000, Mexico.
The authors declare that they have no competing interests.
No funding or grant support was received for this study.
MAQR, study conception, writing the manuscript, dataset interpretation, statistical analysis interpretation, final revision, conclusions; EAQG, figures artwork, tables, photographic material compilation and graphics; MAQG, assistant surgeon; ARA, statistical analysis, tables, graphs; MM, photographic material compilation, final revision; VLG, statistical analysis, final revision. All authors have approved the manuscript for submission.
All co-authors are affiliated to the Faculty of Medicine in the Postgraduate Studies Division at NATIONAL AUTONOMOUS UNIVERSITY OF MEXICO, Mexico City, Mexico.
We express our deep appreciation to the technical staff of Retina Specialists Unit at Oftalmologia Integral ABC, Mexico City. Mexico, which is affiliated to Postgraduate Division studies at National Autonomous University of Mexico. Images a to d and images i to l on Figure 1; images j to l on Figure 2; images a to b and images d to l on Figure 3; images a, b and d to e and h to I on Figure 4; images a, d and g on Figure 5 were generously contributed by Alejandra Nieto-Jordan, Jennifer H. Kim-Lee, Felipe Correa-Esparza and the retina service of the Institute of Ophthalmology. Our special gratitude to Jorge Morales-Navarro for his valuable statistical contribution. Valuable academic contributions were made by Alejandra Nieto-Jordan, Felipe Esparza-Correa and Boris Moreno-Andrade, our recognition and gratitude. All the patients were operated on both campuses of the Centro Medico ABC and Institute of Ophthalmology operating room facilities.