To evaluate the intraocular pressure (IOP)-lowering effect of ripasudil hydrochloride hydrate and the reasons for the discontinuation of its use in glaucomatous eyes with at least 2 ocular hypotensive medications.
Retrospective case series.
We reviewed the medical records of 116 consecutive patients with primary open angle, secondary, or developmental glaucoma. The pre- and post-application IOP up to 6 months after the initiation of ripasudil application was compared after adjusted by the last observation carried forward method. Multivariate analyses were used to identify factors that could account for the IOP-lowering effect of ripasudil. The reasons for discontinuation were evaluated.
The median pre- and post-application IOP at 1, 3, and 6 months was 19.0, 17.5, 17.0, and 16.0 mmHg, respectively. IOP was significantly reduced after ripasudil application at all time points (P < 0.00001, the mixed effect model). The pre-application IOP and glaucoma type were associated with the IOP-lowering effect of ripasudil. Twenty-seven and 8 patients discontinued the application because of unsatisfactory IOP reduction and intolerable side effects, respectively.
Ripasudil reduced IOP in some patients with multiple ocular hypotensives but was discontinued in a fraction of patients due to unsatisfactory IOP reduction and intolerable side effects.
Reduction of intraocular pressure (IOP) is the solely established, interventional treatment modality for glaucoma and is often medically manipulated irrespective of glaucoma type [1]. Ocular hypotensive eyedrops that are commonly used include prostaglandin analog, which enhances uveoscleral outflow, adrenergic β-blocker and carbonic anhydrase inhibitor (CAI), which reduce aqueous humor production, or adrenergic α2 stimulant, which performs both mechanisms. The clinical application of drugs that modify the conventional outflow pathway through trabecular meshwork and the Schlemm canal has been limited, except for pilocarpine for pupillary block release, because of its insufficient IOP-lowering ability. As the Ocular Hypertension Treatment Study revealed, more than 2 agents with different IOP-lowering mechanisms are often required for many patients with glaucoma to achieve their target IOP [2]. However, it has been practically impossible thus far to combine medications of enhanced conventional outflow with other anti-glaucoma eyedrops that enhance the conventional outflow for long-term IOP control because of the lack of an appropriate agent.
Ripasudil hydrochloride hydrate, the newly developed rho-associated coiled-coil forming kinase (ROCK) inhibitor, has been available in Japan since December 2014, and it reduces IOP by enhancing conventional aqueous humor outflow via changes in the morphology of trabecular meshwork cells, inhibition of extracellular matrix production, and increased juxta-Schlemm canal permeability [3-6]. Clinical trials demonstrated that ripasudil eyedrops at 0.4% concentration significantly lowered IOP in normal subjects [7] and in patients with primary open angle glaucoma (POAG) and ocular hypertension [8-10]. However, the clinical trials evaluated the additive effect of ripasudil only in patients with latanoprost or 0.5% timolol [11] and did not test the efficacy of ripasudil in patients with secondary and developmental glaucoma. Furthermore, given the relatively short-term reaction time of ripasudil, the clinical trials measured IOP twice per visit, that is, peak IOP measured 2 hours after instillation and trough IOP measured just before instillation, which were analyzed separately [7-11]. However, it is not practical to measure IOP twice a day or schedule the interval between ripasudil instillation and IOP measurement for many patients in actual clinical practice. There are few studies evaluating the overall IOP-lowering effect of ripasudil eyedrops in such a situation.
The purpose of this study was to evaluate the additive or switching effect of ripasudil in terms of overall IOP reduction in Japanese patients with a variety of glaucoma types and previously receiving at least 2 unspecified anti-glaucoma medications, as well as the reasons for discontinuation in such patients [12].
This is a retrospective case series. The study protocol was approved by the institutional review board of Kobe University (No. 270023; UMIN000025466), and adhered to the tenets of the Declaration of Helsinki. We obtained oral consent from all patients, or from legal guardians for patients under 20 years of age, after the study purpose and design were fully explained and noted on the medical records.
We reviewed the medical records of 116 consecutive glaucoma patients who were not under their own target IOP, despite using at least 2 preexisting anti-glaucoma medications, met the inclusion and exclusion criteria described below, and received ripasudil for the first time during December 2014, when ripasudil was on the market, and November 2015 in the outpatient clinic section of the Kobe University hospital. There was no restriction based on patient age, glaucoma type, number of anti-glaucoma medications, or intervals between ripasudil instillation and IOP measurement, in the initiation of the ripasudil medication.
Patients with normal tension glaucoma were included into those with POAG. Patients were defined as having developmental glaucoma if they had the onset of disease under 18 years of age, a history of elevated IOP exceeding 21 mmHg, if necessary, under general anesthesia, abnormal corneal signs such as opacity, enlargement of diameter, and the presence of Haab's striae in the case of children under 3 years, or glaucomatous optic nerve and visual field changes similar to POAG in the case of older children. Patients were defined as having secondary glaucoma if they had elevated IOP exceeding 21 mmHg before ripasudil was initiated, and specified etiologies for IOP elevation such as pseudoexfoliation, intraocular inflammation, and local or systemic steroid administration. However, patients who had changed the regimen or content, if any, of the steroid therapy between 3 months prior to and 6 months after the initiation of ripasudil medication were excluded from the study entry, because such changes may have influenced the IOP control. The presence of glaucomatous optic nerve changes was not used as a criterion in this group.
Inclusion criterion for analysis was an eye with higher IOP in patients who bilaterally received ripasudil or in the right eye in patients whose IOP was the same in both eyes. In other words, 1 eye per patient was included in the analysis. Exclusion criteria were eyes that (1) underwent laser or intraocular surgeries within 6 months before the start of ripasudil treatment and (2) could not have IOP measured by the Goldmann applanation tonometer.
Full ophthalmic examinations were conducted in all 116 patients within 3 months before the initiation of ripasudil, which included best-corrected decimal visual acuity, slit-lamp biomicroscopy, anterior chamber angle examination, stereo-fundoscopy under pupillary dilation, and central corneal thickness measurement using a specular microscope (Noncon Robo SP-8000; Konan Medical, Tokyo, Japan). The average IOP calculated from 2 measurements immediately before initiation of ripasudil medication was defined as the premedication IOP, whereas the single IOP measurement per visit at 1, 3, and 6 months was the post-medication IOP at each visit. The premedication prescription score was calculated as 1 for any anti-glaucoma eyedrops and 2 for oral CAI.
Continuous variables were expressed as means ± SDs if they followed the Gaussian distribution or medians (interquartile ranges) if they did not.
Pre-medication IOP and IOP measured at 1, 3, and 6 months after initiation of ripasudil per patient were compared using the mixed effect model in two ways. First, the comparisons were made among IOP data collected from patients who completed follow up until 6 months after initiation of ripasudil application. Second, the comparisons were made among IOP data adjusted by the last observation carried forward (LOCF) method [13]. In other words, missing data from patients who discontinued the ripasudil medication by 6 months after ripasudil application were imputed with the LOCF method.
Intergroup comparisons for continuous variables were made using an unpaired t-test or one-way analysis of variance (ANOVA), followed by the Tukey-Kramer test as a post hoc test, if they followed the Gaussian distribution, and by Mann-Whitney U-test or Kruskal-Wallis test if they did not follow the Gaussian distribution. Intergroup comparisons for nominal variables were made by chi-square test. Multiple regression analysis was conducted to investigate which explanatory variables could account for the LOCF-adjusted IOP reduction rate at 6 months after the initiation of ripasudil application. The explanatory variables included age, sex, glaucoma type, central corneal thickness, premedication IOP, number of previous glaucoma-related surgeries, and number of premedication prescription scores.
The statistical analysis was performed using MedCalc (version 10.4.0, MedCalc Software, Mariakerte, Belgium) and SPSS Statistics (version 24, IBM Japan, Tokyo, Japan). A P value of less than 0.05 was considered statistically significant.
Demographic data of the 116 cases are listed in Table 1. The glaucoma type was POAG in 76 eyes, secondary glaucoma in 31, and developmental glaucoma in 9. The etiology of secondary glaucoma included pseudoexfoliation in 15 eyes, uveitis in 9, steroid-induced in 4, avitreous-related in 2, and nanophthalmos-related in 1. The premedication prescription score was 2 in 23 eyes, 3 in 46, 4 in 40, and 5 in 7, the median of which was 3.
Table 1: Clinical background of all patients. View Table 1
Figure 1 illustrates the time course of changes in IOP of whole patients. As shown in Figure 1A, the median (interquartile range) premedication and post-medication IOP at 1, 3, and 6 months after the initiation of ripasudil application in patients who completed the follow-up until 6 months (n = 81) was 20.0 (17.0~23.0), 18.0 (15.0~20.0), 17.0 (15.0~19.0), and 16.0 (14.0~19.0), respectively, mmHg. The post-medication IOP was significantly lower at all 3-time points than the premedication IOP (the mixed effect model; P < 0.00001). When adjusted by the LOCF method (n = 116), these values were 19.0 (17.0~22.5), 17.5 (15.0~22.0), 17.0 (15.0~20.0), and 16.0 (15.0~20.0), respectively, mmHg (Figure 1B). Again, the post-medication IOP was significantly lower at all 3-time points than the premedication IOP (the mixed effect model; P < 0.00001).
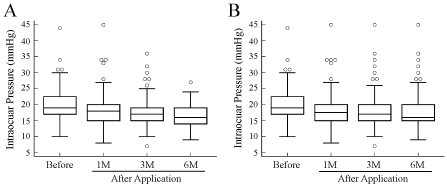 Figure 1: Box plots of time-course changes in intraocular pressure before and after the application of ripasudil hydrochloride hydrate.
Figure 1: Box plots of time-course changes in intraocular pressure before and after the application of ripasudil hydrochloride hydrate.
A) Data of 81 out of 116 enrolled patients, who completed the follow-up until 6 months (M) after the initiation of ripasudil instillation; B) Data of all 116 patients, which were adjusted by the last observation carried forward method. Open circles indicate outside values defined as values that are smaller than the lower quartile minus 1.5 times the interquartile range, or larger than the upper quartile plus 1.5 times the interquartile range. View Figure 1
At 6 months, 49 eyes showed a 15% or greater IOP reduction rate, whereas the remaining 67 eyes either had an IOP reduction rate less than 15% (n = 32) or discontinued the use of ripasudil due to unsatisfactory IOP control or severe local side effects (n = 35).
The clinical background of each glaucoma type is listed in Table 2. There were no differences in background except for age among three subgroups.
Table 2: Comparison of demographics among three groups of patients. View Table 2
Due to a small sample size of the developmental glaucoma subgroup, the time-course changes in IOP were analyzed only in patients with POAG and secondary glaucoma as illustrated in Figure 2. In the case of POAG, the pre-and post-medication IOP at 1, 3, and 6 months after the initiation of ripasudil application in the patients who completed follow-up (n = 51) was 20.0 (17.0~22.0), 16.0 (15.0~19.0), 16.0 (14.0~19.0), and 16.0 (14.0~18.75), respectively, mmHg (Figure 2A). When adjusted by the LOCF method (n = 76), these values were 18.5 (17.0~22.0), 17.0 (15.0~19.0), 16.0 (14.0~18.0), and 16.0 (14.5~18.0), respectively, mmHg (Figure 2B). In the case of secondary glaucoma, the pre-medication and post-medication IOPs at 1, 3, and 6 months were 22.0 (19.0~27.0), 17.5 (14.5~20.0), 16.5 (13.5~20.5), and 16.0 (14.0~20.0) mmHg, respectively, when patients who completed the follow-up were examined (n = 16) (Figure 2A). These values were 21.0 (17.25~26.0), 18.0 (14.5~22.0), 19.0 (16.0~21.75), and 19.0 (16.0~22.0) mmHg, respectively, when the LOCF method was used (n = 31) (Figure 2B). The post-application IOP at any time point was significantly lower than the pre-application IOP in eyes both with POAG and secondary glaucoma (the mixed effect model; P < 0.00001 and P = 0.0002 for POAG and secondary glaucoma, respectively) when the complete users were analyzed (Figure 2A). In contrast, this held true for patients with POAG even when the LOCF method was applied (P < 0.00001), while there was no significant difference in IOP among four test points in patients with secondary glaucoma (P = 0.06) when the LOCF method was applied (Figure 2B).
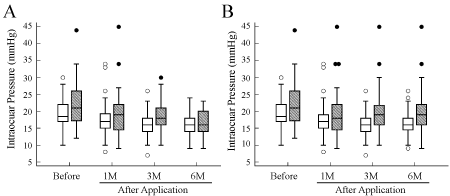 Figure 2: Box plots of time-course changes in intraocular pressure before and after the application of ripasudil hydrochloride hydrate in patients with primary open-angle glaucoma (POAG) and secondary glaucoma.
Figure 2: Box plots of time-course changes in intraocular pressure before and after the application of ripasudil hydrochloride hydrate in patients with primary open-angle glaucoma (POAG) and secondary glaucoma.
A) Data of patients who completed the follow-up until 6 months after the initiation of ripasudil instillation; B) Data of all 107 patients, which were adjusted by the last observation carried forward (LOCF) method. Open solid box, POAG; shaded box, secondary glaucoma. Open and closed circles indicate values that are smaller than the lower quartile minus 1.5 times the interquartile range or larger than the upper quartile plus 1.5 times the interquartile range, in patients with POAG and secondary glaucoma, respectively. View Figure 2
Figure 3 shows histograms of the number of patients either with POAG (Figure 3A) or secondary and developmental glaucoma (Figure 3B) stratified by the magnitude of IOP reduction at 6 months relative to pre-medication IOP when the LOCF method was applied. The patients with POAG exhibited a skewed distribution toward the right, whereas those with secondary and developmental glaucoma showed a normal distribution, with the former having a narrower range than the latter.
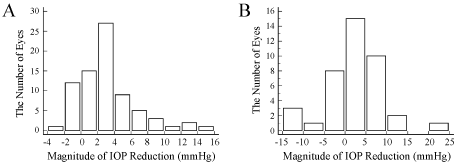 Figure 3: Distribution of patients stratified by the magnitude of intraocular pressure (IOP) reduction at 6 months relative to pre-medication IOP.
Figure 3: Distribution of patients stratified by the magnitude of intraocular pressure (IOP) reduction at 6 months relative to pre-medication IOP.
A) The IOP was adjusted by the last observation carried forward test. A. Primary open angle glaucoma; B) Secondary or developmental glaucoma. View Figure 3
Figure 4 depicts the scatter plots of IOP at final evaluation and IOP reduction rates against IOP before ripasudil treatment, in which the IOP was adjusted by the LOCF method. The IOPs at the final evaluation was below those before ripasudil treatment in most patients with POAG, whereas the distribution of IOPs were broad in patients with secondary and developmental glaucoma and, so, some patients with secondary and developmental glaucoma showed exhibited even higher IOP at the final evaluation than the pre-medication IOP (Figure 4A).
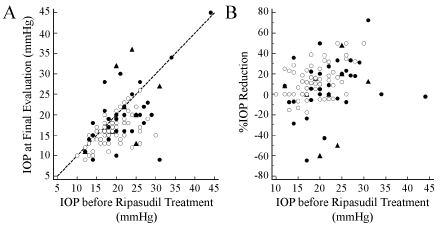 Figure 4: Scatter plots of intraocular pressure (IOP) at final evaluation (A) and IOP reduction rate (B) against IOP before ripasudil treatment.
Figure 4: Scatter plots of intraocular pressure (IOP) at final evaluation (A) and IOP reduction rate (B) against IOP before ripasudil treatment.
The IOP was adjusted by the last observation carried forward method. Open circles, primary open-angle glaucoma; closed circles, secondary glaucoma; triangles, developmental glaucoma. View Figure 4
Patients with POAG demonstrated a significant positive correlation between the pre-medication IOP and the IOP reduction rate (correlation coefficient, 0.48; P < 0.0001). In comparison, patients with secondary glaucoma and developmental glaucoma did not show significant correlation between the two variables (P = 0.12 for secondary glaucoma, P = 0.14 for developmental glaucoma) (Figure 4B).
The multiple regression analysis demonstrated that the glaucoma type (partial correlation coefficient -0.30, P = 0.002) and the pre-medication IOP (partial correlation coefficient 0.40 P < 0.0001) were the factors among the tested parameters that could account for the IOP reduction rate at 6 months after the initiation of ripasudil (adjusted coefficient of determination 0.175). Age, sex, central corneal thickness, pre-medication prescription score, and the number of previous surgeries could not account for the IOP reduction rate.
Table 3 summarizes reasons for the 35 patients who discontinued the ripasudil instillation by 6 months. Of the 35 cases, 27 eyes showed poor IOP control, resulting in either additional glaucoma surgery or switching from ripasudil to other medications, whereas the remaining 8 eyes discontinued ripasudil medication because of adverse effects. The pre-medication IOP, age, sex, central corneal thickness, the median pre-medication prescription score, and the mean deviation in this group was 20.2 ± 6.2 mmHg (P = 0.79), 61.3 ± 18.4 years (P = 0.78), male/female (16/19; P = 0.29), 517.1 ± 38.0 μm (P = 0.95), 3 (P = 0.20), and 13.32 ± 7.98 dB (P = 0.239), respectively, all of which were statistically insignificant compared with the corresponding parameters of the complete users during the entire tested periods of 6 month. The discontinuation group consisted of POAG in 16 eyes, secondary glaucoma in 17 (uveitis in 7, pseudoexfoliation in 5, steroid-induced in 4, avitreous-related in 1), and developmental glaucoma in 2. Therefore, when developmental glaucoma was excluded from analysis due to the small sample size, the discontinuation group had a significantly higher proportion of secondary glaucoma in glaucoma type compared with the complete user group (chi-square test; P = 0.005).
Table 3: Reasons for discontinuation of ripasudil prescription. View Table 3
In this study, the IOP-lowering effect of ripasudil 0.4% eyedrops was analyzed in a total of 116 eyes with a variety of glaucoma types that had already used at least 2 preexisting anti-glaucoma medications. As a result, approximately 70% (= 81) patients continuously used ripasudil until 6 months after the initiation of its use, whereas 30% (= 35 eyes) patients discontinued its use due to unsatisfactory IOP control or intolerable side effects. Further, 49 eyes (= 42.2% of total and 60.4% among the complete users) exhibited a 15% or greater IOP reduction rate. The IOP reduction rate was significantly associated with the pre-medication IOP and glaucoma type; i.e., the eyes with higher pre-medication IOP or/and POAG were more likely to show higher IOP reduction rate at 6 months after the initiation of ripasudil instillation. The response to ripasudil was more variable in patients with secondary and developmental glaucoma compared with patients with POAG.
There are, in the literature, 5 reports of clinical trials [7-11] and 4 reports of postmarketing clinical studies [13-16] regarding the IOP-lowering effect of ripasudil with 0.4% concentration. In terms of ripasudil monotherapy, the phase I clinical trial demonstrated that the single or 7-day instillation of ripasudil showed a 4-mmHg reduction of IOP 2 hours after the instillation in controls [7]. The phase II trials targeting patients with POAG or ocular hypertension showed that the magnitude of IOP reduction after an 8-week instillation was 3.5 mmHg at trough (IOP measured immediately before instillation), and 4.5 mmHg at peak (IOP measured 2 hours after the last instillation) [8]. The average IOP reduction after a 52-week instillation was 2.6 mmHg at the trough level and 3.7 mmHg at the peak level [10]. According to other clinical trial reports, the additive effect of ripasudil on IOP reduction in eyes with 0.5% timolol was 2.2 mmHg at the trough level and 3.0 mmHg at the peak level, while that in eyes with latanoprost was 1.4 mmHg and 2.4 mmHg, respectively [11]. The additive effect of ripasudil in those with a fixed combination of 0.5% timolol and latanoprost was 1.7 mmHg at both the trough and peak levels [11].
In contrast, the 4 post-marketing clinical research studies evaluated the additive effect of ripasudil in eyes treated with multiple anti-glaucoma medications [13-16]. A prospective case series study by Inazaki, et al. recruited 35 patients who had POAG and poor IOP control under maximally tolerated medications and found that the addition of ripasudil for 3 months resulted in a 2.8 mmHg IOP reduction on average [13], which is similar to our result of the magnitude of IOP reduction in patients with POAG being 2 mmHg. Similarly, Inoue, et al. found the additive IOP-lowering effect of ripasudil in a total of 119 eyes of 119 patients with POAG or ocular hypertension, who had already used two or more anti-glaucoma medications [14]. In their study, the average IOP before the initiation of ripasudil application was 19.8 mmHg, while the IOP at 1 month and 3 months after the initiation was 17.5 and 16.8 mmHg, respectively [14]. The present results of patients with POAG were also in agreement with their study. Such an additive effect of ripasudil may be attributed to the distinct pharmacological action of ripasudil of the conventional aqueous humor outflow modification. The observation that ripasudil was more effective in eyes with POAG and a higher pre-medication IOP may be accounted for by the fact that the conventional outflow is known to be IOP-dependent [17].
A retrospective study by Sato, et al. analyzed the IOP-lowering effect of ripasudil in a total of 92 patients with various types of glaucoma under maximally tolerated medications, which disclosed that the addition of ripasudil led to a 1.4 mmHg IOP reduction at 6 months on average [15]. The present findings of median IOP reduction of 2 or 1 mmHg in patients with POAG or those with secondary and developmental glaucoma are comparable for their result. According to Sato, et al. the IOP in eyes with developmental glaucoma and secondary glaucoma other than exfoliation glaucoma was reduced more than the IOP in eyes with POAG and NTG [15]. On the contrary, when the LOCF method was applied, the IOP reduction after the initiation of ripasudil instillation was marginal in patients with secondary glaucoma in the present study. One possible reason for this is that the response to ripasudil was so variable in patients with secondary and developmental glaucoma, compared with those with POAG (Figure 3 and Figure 4), that the statistical power may have been eliminated. Alternatively, the LOCF method in this study evaluated the whole patients' data, which included the discontinuation group and may have biased the data toward a negative result, whereas the study by Sato, et al. [15] did not adjust the data although only 55 eyes completed the follow-up of 6 months, which may have biased the data toward positive result. More recently, Matsumura and colleagues [16] demonstrated that adding ripasudil to prostaglandin analogue significantly reduced IOP in 27 eyes of 16 patients with exfoliation glaucoma from 16.2 mmHg before ripasudil application to 13.1 mmHg at 5 or 6 months after the ripasudil application. The high IOP-lowering effect of ripasudil achieved in eyes with exfoliation glaucoma in their study is thought to be due to the second, instead of third or higher order, line use of ripasudil.
Inazaki, et al. reported that the proportion of the eyes in which ripasudil medication achieved the target IOP was at most 48.5% [13], whereas 30% (= 35 eyes) patients discontinued its use due to unsatisfactory IOP control or intolerable side effects in the present study. Although there was no significant difference in the premedication IOP, the number of preexisting ocular hypotensives used, age, sex, central corneal thickness, or the severity of visual field defect between the complete users and patients discontinued the ripasudil instillation, the discontinuation group had a higher proportion of secondary glaucoma among glaucoma types. This suggests that ripasudil may have less ability to lower IOP in eyes with secondary glaucoma. Alternatively, the eyes with secondary glaucoma may have been more likely to undergo glaucoma surgery earlier than those with POAG, because eyes with exfoliation glaucoma, which was included in the secondary glaucoma subgroup, are known to have a faster progression compared with POAG [18], or glaucoma surgery may have been performed when uveitis was inactive. However, more cases are needed to address this issue, because the subjects in this study probably included the eyes with the trough IOP measurement due to random sampling as mentioned previously.
Taken together with these studies and ours, it seems reasonable to state that ripasudil has a potency to reduce IOP in patients with POAG and treated with multiple ocular hypotensives, while further studies are needed to address whether ripasudil could be also effective in eyes with secondary or developmental glaucoma, particularly in those treated with multiple anti-glaucoma medications.
There were 8 eyes in which ripasudil treatment was discontinued due to local adverse effects, although there were no cases of systemic side effects. Given that vascular dilation is one of the pharmacological actions of ROCK inhibitors [19], the clinical trials and the post-marketing clinical studies reported the frequency of occurrence of conjunctival injection ranging from 57.4% to 100%. However, in this study, the main reasons for dropping out were allergic conjunctivitis and blepharitis. Further longitudinal evaluation is necessary to elucidate to what extent such events increase along with the longer-term application of ripasudil.
Limitations of this study were (1) The small sample size of each glaucoma type, particularly that of developmental glaucoma, (2) Incomplete access to data regarding steroid therapy regimen in the secondary glaucoma group due to the retrospective design, (3) No evaluation of placebo effect, (4) The possibility that the doctors used their discretion in determining whether or not ripasudil was used for 6 months may have influenced the average IOP reduction rate, (5) The possible underestimation of adverse effects because the most serious one was selected among multiple side effects in the same individuals and because mild ones that did not lead to dropouts were ignored.
The IOP-lowering effect of ripasudil hydrochloride hydrate and the reasons for discontinuation were retrospectively evaluated in 116 patients who had a variety of glaucoma types that already used at least 2 glaucoma medications. The median magnitude of IOP reduction at 6 months was 2.0 mmHg. The higher pre-application IOP and POAG among glaucoma type were associated with higher IOP reduction. Unsatisfactory IOP control or intolerable side effects led 30% patients to discontinue its use. Ripasudil can reduce IOP even in some patients who have already used multiple anti-glaucoma medications.
This study was partly supported by JSPS KAKENHI 20592043 (MN, YY, AN). MN received unrestricted research funding from Kowa, Ltd.
English was edited by Crimson Interactive Pvt. Ltd.
COI: None of the authors has personal financial interest relevant to this study. The authors alone are responsible for the content and writing of the paper.
Clinical trial registration No.: UMIN000025466.