Trans-spinal Direct Current Stimulation (tsDCS) can alleviate pain perception in humans through plastic processes. Brain Derived Neurotrophic Factor (BDNF) has been shown to influence a large number of physiological processes including those underpinning neural plasticity. A common polymorphism of BDNF (val66met), reduces the efficiency of plastic processes. We studied the effect of this BDNF polymorphism on the signalling and perception of thermal pain and to what degree these can be influenced by tsDCS in young healthy adults. For those carrying a less optimal form of BDNF, the pain mitigating effect of tsDCS was reduced. BDNF typing may be used to improve individualization in pain treatment using tsDCS, thereby reducing the variability regarding the effect of this technique.
BDNF, tsDCS, Pain
We describe the effect of polymorphism of the plasticity promoting protein Brain Derived Neurotrophic Factor (BDNF) on the signalling and perception of thermal pain and to what degree these can be influenced by trans-spinal Direct Current Stimulation (tsDCS) in healthy adults.
Early studies [1] indicated that anodal tsDCS can alleviate pain perception in humans. We recently expanded and corroborated these findings in a study (placebo control, double blind, crossover design [2]), demonstrating that at the group level, anodal tsDCS alleviates pain perception in parallel to reduced pain signalling (evaluated by laser evoked potentials) in healthy adults. However, as for most studies of neuromodulation, the interindividual variance was large.
The clinical usefulness of a treatment option is decided by many factors. Aspects favouring tsDCS are its virtually complete lack of side effects, low price and simplicity possibly allowing wide home use [3]. Even so, it would gain the introduction of the technique into clinical practice if those having negligible chances of benefitting, could be easily identified prior to actual treatment. BDNF has been shown to influence a large number of physiological processes including those underpinning neural plasticity [4]. BDNF appears in two common single nucleotide polymorphism (SNP) forms with, in position 66, valine in both- or methionine in one or both positions. The two latter forms (combined prevalence ca 35 percent among Caucasians) [5] lead to reduced neural plasticity as investigated in various systems including the spinal cord [6]. Our aim was to study, at an individual level, whether there is a relationship between the form of BDNF present and the objective and subjective effects of tsDCS on pain perception and signalling, respectively.
Details of the subjects studied, and methods used were recently presented [2]. Briefly, nineteen healthy young subjects, (24 ± 3-years-old, ♂/♀; 10/9) were studied using a randomized, double-blind, placebo controlled cross over design. Heat pain stimulation of one foot was performed using a laser stimulus that was likewise the stimulus for the assessment of laser evoked potentials (LEP). The participants rated the subjectively perceived pain using a visual analogue scale. These parameters were assessed before- (BL), at the end of- (T0) and 30 minutes after (T30) 20 minutes of tsDCS (2.5 mA with the anode at Th10 and the cathode at a shoulder). Of the nineteen original subjects, five were lost to this follow up; three could not be reached, for two the LEP signals could not be analyzed. We have no reason to believe that these five subjects differed from the rest.
Regarding the analysis of BDNF type, buccal cells were captured with a rinse of phosphate buffered saline. Cells were concentrated by 3000g centrifugation and DNA extracted following proteinase K digestion and ethanol precipitation. Samples were genotyped by PCR amplification using an established protocol [7] and analyzed by agarose gel electrophoresis.
Of the 14 subjects, two were heterozygotic (val66met), none were homozygotic. At the group level, the subjects of the two groups differed regarding effects of tsDCS on both pain perception and pain signaling (Figure 1). At the individual level, we used a reduction of pain perception of 30 per cent (conventional level) or a prolongation of LEP latency of 5 per cent (arbitrary level) as a cut off indicating a significant effect of tsDCS stimulation on pain perception and pain signalling, respectively. Using these criteria, of those having a more optimal BDNF type, 9/12 were responders and 6/12 had a latency prolongation. For those with a less optimal BDNF type, the corresponding numbers were 0/2 and 1/2. Thus, from the opposite angle, all responders had a favorable BDNF type.
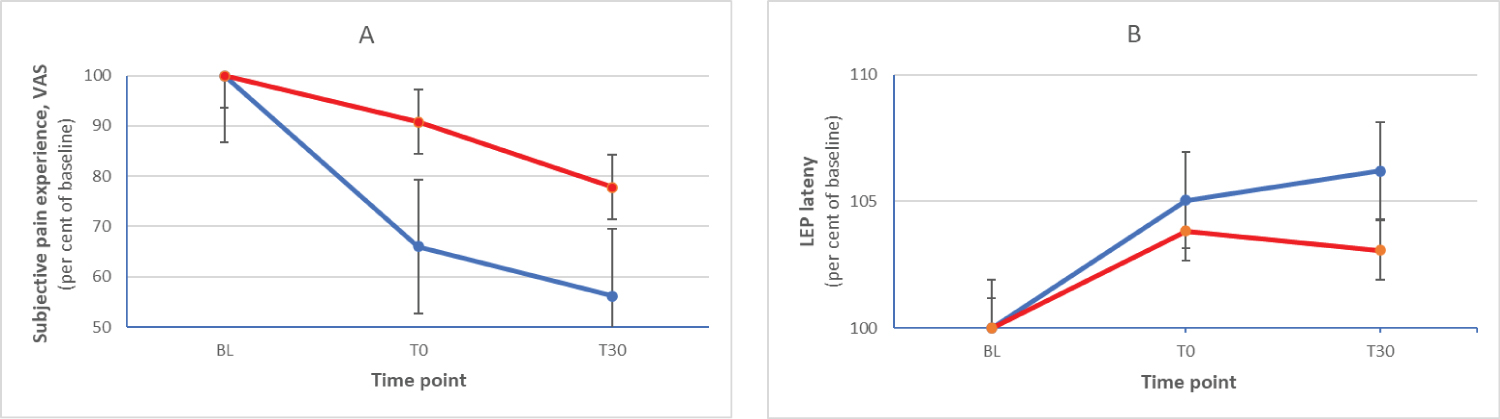 Figure 1: Relative changes (mean and standard deviation, per cent of baseline) of subjective pain experience (A) and LEP latency (B) at the three time points for the group of subjects with val66val (blue) and val66met (red) BDNF.
View Figure 1
Figure 1: Relative changes (mean and standard deviation, per cent of baseline) of subjective pain experience (A) and LEP latency (B) at the three time points for the group of subjects with val66val (blue) and val66met (red) BDNF.
View Figure 1
In a somewhat restricted number of healthy young subjects, we found that BDNF type affects the capacity of tsDCS to diminish afferent pain signalling and to reduce pain perception.
Neural plasticity can be seen as a double-edged sword. On the one hand, it is essential for the fundamental capacity of the CNS to adapt to changes that optimizes the possibilities for the organism, on the other, it may lead to so called maladaptive plasticity that can be part of dysfunctional processes such as sensitization in neuropathic pain [8]. Regarding BDNF, this may mean that those carrying the val66val form can benefit from more optimal underpinnings to learning processes but also a higher risk of developing chronic pain [8]. Generally, in plastic processes, early changes of activity are mainly based on alterations of function (e.g. transmitter release) and later, more on structural modifications (e.g. stabilization of newly developed axonal contacts). Possibly, there may be an interaction between the influence of, on the one hand, degree of plastic capacity and, on the other, the duration of the dysfunction inducing the changes. If so, in the case of BDNF polymorphism, people with the val66val form compared to those with val66met, might have a higher chance of benefitting from neuromodulation early- (mainly functional changes), but a lower chance late (mainly structural changes) in the process.
From a physiologic point of view, the capacity of tsDCS as applied in this study to relieve pain is dependent on the BDNF genotype of the subject. From a clinical perspective, pain relief may be achieved in a majority of subjects with the most common (val66val) type. For those with a methionine substitution, alternative measures seem to be needed.
This work was supported by ALF Grants, Region Östergötland, the Swedish Society of Medicine, the Medical Research Council of Southeast Sweden, the Östergötland County Council, the Linköping Society of Medicine and the Knut and Alice Wallenberg foundation.
None of these bodies had any influence on decision on study design, collection, analysis or interpretation of data, writing of- or decision to submit the report.
None.