Autonomic Regulation Therapy (ART) using chronic Vagus Nerve Stimulation (VNS) is in development for patients with heart failure who remain symptomatic despite standard care. In the ANTHEM-HF Pilot Study, a therapeutic VNS intensity was successfully achieved in patients with HFrEF using manually programmed VNS up-titration. An algorithm has been developed for automatically intensifying VNS in small increments to a programmable target intensity, and capable of adjustments during the up-titration process using a hand-held programmer or magnet.
Six healthy canines were implanted with a pulse generator and lead for right cervical VNS. Device programming at implant activated the titration algorithm with (1) A 1-week start delay; (2) Initial stimulation intensity of 0.125 mA, 130 μsec pulse width, and 5 Hz frequency; (3) Target stimulation intensity of 2.5 mA, 250 μsec pulse width, and 5 Hz frequency; and (4) Trajectory to achieve the target intensity in approximately 10 weeks. Magnet placement over the pulse generator at scheduled intervals tested algorithm design intention to decrement VNS intensity, prolong the time to reach the target intensity, and/or temporarily inhibit VNS.
All animals underwent successful VNS system implantation and completed all scheduled activities. There was one transient implant-related adverse event (Horner's Syndrome). The "TitrationAssist" algorithm performed as designed. The targeted VNS intensity was achieved as scheduled and automated titration was well-tolerated in all animals. There were no stimulation-related adverse events. ECG monitoring demonstrated no clinically significant cardiac findings. Detailed gross necropsy and macroscopic examinations revealed vagus nerves and all major organs to be normal in all animals.
An implantable VNS device with Titration Assist algorithm for automatically increasing VNS to a target intensity, and capable of adjustment in response to specific magnet applications, was successfully tested in a preclinical study of healthy canines. Titration Assist performed as designed appears to be safe and could dramatically reduce the burden of titration in a clinical setting.
Autonomic regulation of cardiac function is a complex process involving hierarchical interactions among central and peripheral neural networks that are comprised of sympathetic and parasympathetic elements of the autonomic nervous system [1]. Ordinarily, the sympathetic and parasympathetic nervous systems work together to tightly regulate appropriate cardiovascular function under a variety of environmental conditions. In chronic heart failure (HF), as heart pumping function becomes impaired, the autonomic nervous system attempts to compensate by increasing sympathetic activation and reducing central outflow of parasympathetic activity [2]. Initially, this helps maintain cardiac output; however, prolonged sympathetic hyper activation is associated with maladaptive cardiovascular responses that promote HF progression and worse outcomes [3,4].
The VITARIA® Therapy System (LivaNova PLC, London, UK) is a CE Marked implantable device that delivers Autonomic Regulation Therapy (ART) via Vagus Nerve Stimulation (VNS) in patients with chronic HF and reduced ejection fraction (HFrEF). ART increases parasympathetic activity, counteracts sympathetic hyperactivation, and helps to restore autonomic balance. In pilot clinical studies, ART has been associated with improved cardiac function and reduced HF symptoms in patients with chronic HFrEF [5,6].
Because patients are unable to tolerate the sudden initiation of the intended target intensity for VNS immediately after VNS system implantation, VNS is gradually increased after implantation from a low level over a 10-12 week titration period [7]. This may require the patient to return to the clinic weekly or biweekly for a succession of manual titrations of VNS. This process imposes a significant resource and travel burden on both the patient and on the clinic.
The objective of this study was to evaluate the chronic safety and performance of an automated titration feature (Titration Assist™, LivaNova USA Inc., Houston TX), which can be programmed by the physician to automatically increase stimulation intensity gradually outside of the clinic in a manner that mimics manual titration.
Normal mongrel canines (n = 6) were implanted with the VITARIA System (Figure 1), which consists of a VITARIA™ Model 7304 Lead on the right cervical vagus nerve and a VITARIA Model 7103 Pulse Generator in a subcutaneous pocket in the animal's right dorsolateral neck area. All procedures were performed under general anesthesia and sterile conditions. The study was approved by the NAMSA Institutional Animal Care and Use Committee and conformed to the Food and Drug Administration "Principles of Good Laboratory Practice (GLP)" and the National Institute of Health "Guide and Care for Use of Laboratory Animals."
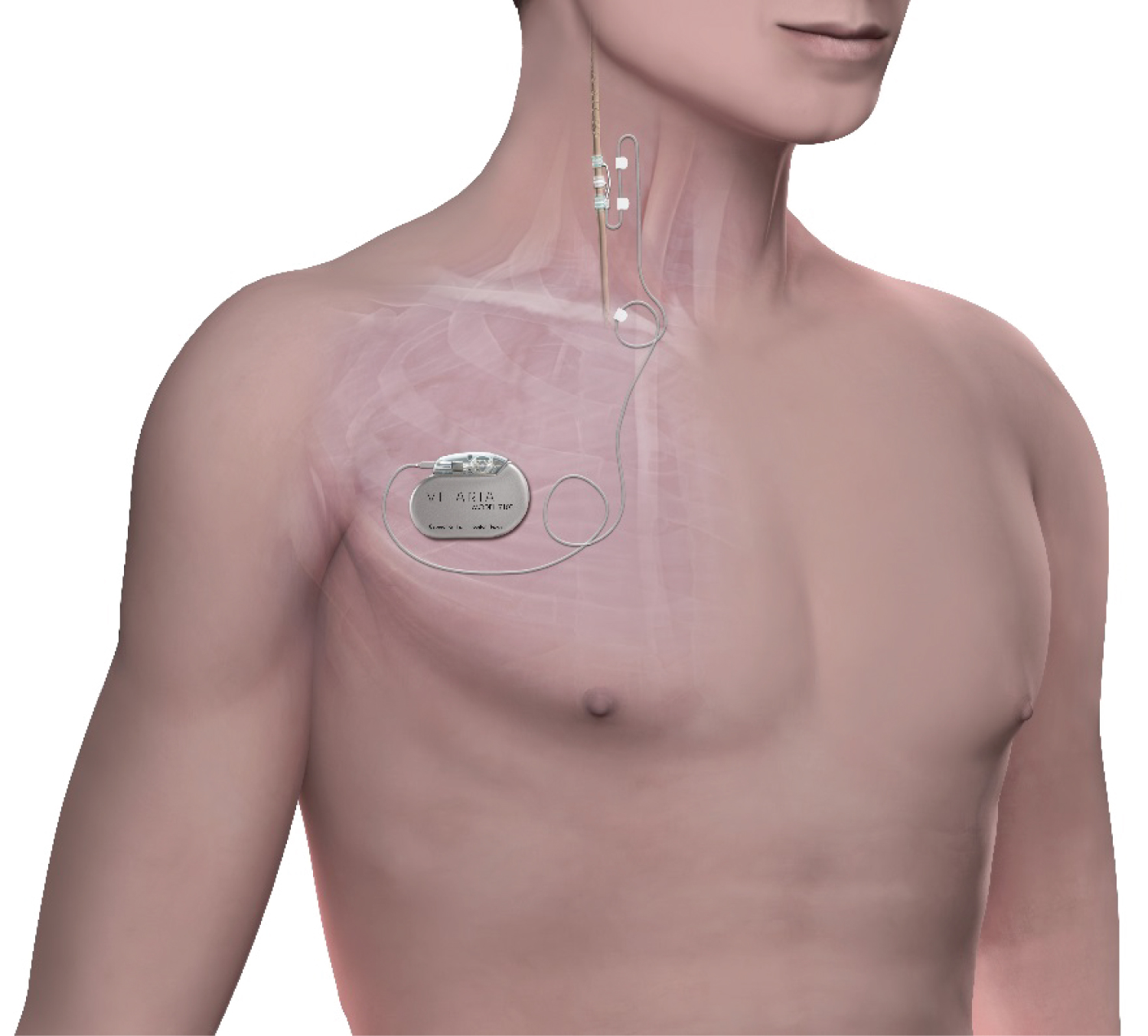 Figure 1: The VITARIA Autonomic Regulation Therapy (ART) system implanted on the right cervical vagus nerve.
View Figure 1
Figure 1: The VITARIA Autonomic Regulation Therapy (ART) system implanted on the right cervical vagus nerve.
View Figure 1
Device interrogations were performed weekly following activation using an external programmer (VITARIA Model 7250). Baseline Holter monitoring and veterinary health assessment were performed prior to implantation and at the end of the titration period. Clinical observations were performed at least daily for the duration of the study. Following initiation of titration, animals were monitored for change in heart rate and presence of arrhythmia. Signs of discomfort (e.g. coughing, cheek puffing, excessive drooling, postural changes, or anxious behavior) were assessed daily. At the end of the study, a gross necropsy was performed for each subject.
Baseline and terminal Holter recordings were analyzed, including a description of any clinically significant change occurring between baseline and terminal time points. Any changes from baseline in the frequency and/or severity of arrhythmias were assessed by a veterinary cardiologist as appropriate to the animal model. An independent test facility veterinarian used this arrhythmia assessment to determine if these changes were related to VNS therapy.
At implant, stimulation was activated at current amplitude of 0.125 mA, a pulse width of 130 μs, a frequency of 5 Hz, an ON-time of 14 seconds, and an OFF-time of 66 seconds. Additionally, the TitrationAssist feature was activated with a one-week delay and target stimulation parameters of 2.5 mA, 250 μs, 5 Hz, 14 sec/66 sec. Once activated, TitrationAssist automatically increased stimulation intensity multiple times a day at programmed increments and rate until the target intensity was reached. Two of the animals were programmed to have stimulation intensity increments occur 4 times per day, and the remaining four animals were programmed with a Sleep Inhibition feature, which suspends stimulation intensity increases during the night and results in 3 intensity increments each day.
Prior to TitrationAssist initiation, stimulation intensity was temporarily increased manually to verify that all animals exhibited an expiratory reflex once stimulation intensity reached the tolerance zone boundary. During automated titration, the Patient Magnet was used to decrease ("rollback") stimulation intensity to confirm proper rollback performance. The rollbacks were performed at 3 weeks after VNS system implantation, and once the animals reached the 2.5 mA amplitude target.
The TitrationAssist stimulation protocol is shown in Figure 2. TitrationAssist was programmed to automatically increase stimulation intensity several times daily using the nominal time progression curve (titration target reached after approximately 45 days). At 2 weeks post-implant, TitrationAssist was reprogrammed to the faster time progression curve (titration target reached after approximately 30 days). The magnet rollback that occurred at 3 weeks reverted the algorithm back to the nominal curve. Once the animals reached the 2.5 mA amplitude target, a second magnet rollback shifted the algorithm to the slower time progression curve (approximately 60 days) for the duration of the study.
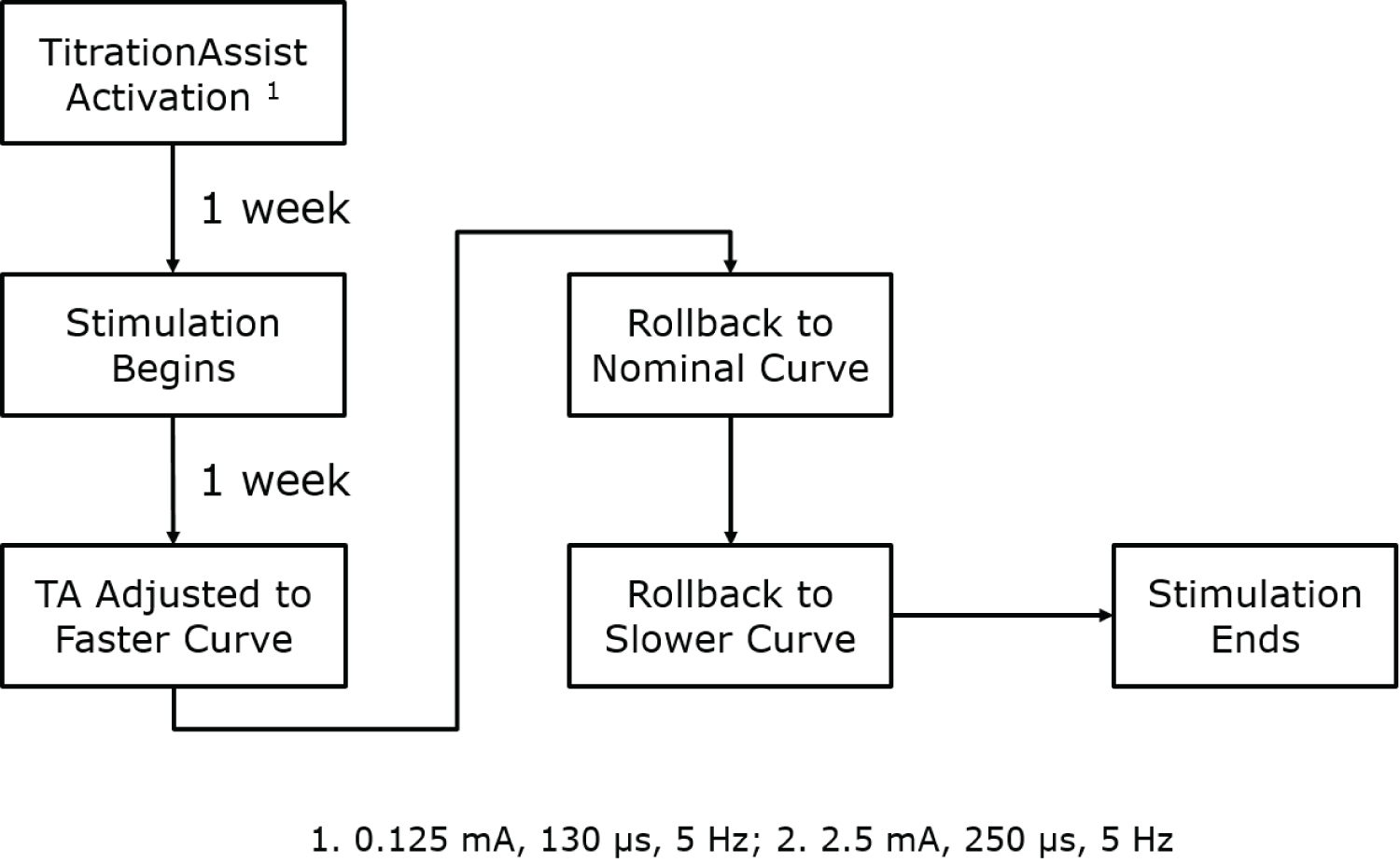 Figure 2: TitrationAssist Timeline.
View Figure 2
Figure 2: TitrationAssist Timeline.
View Figure 2
At the terminal procedure, animals were euthanized by barbiturate overdose. A complete macroscopic examination of the implant sites and major organs was performed on all animals. Both the right vagus nerve with the implanted lead and the contralateral vagus nerve were collected. The subcutaneous pocket, including skin and subcutaneous tissue, and representative sections of major organs (brain, heart, lung, liver, spleen, kidneys, and gastrointestinal tract) were also analyzed.
All animals were successfully implanted with the VITARIA System. The stimulation intensity up to 2.5 mA, 250 μsec, 5 Hz was well tolerated by all animals. None of the animals exhibited any side effects in response to stimulation during the titration period, despite these effects being elicited at the beginning of the titration period in response to manual probing of the tolerance zone boundary. Animals with stimulation increases four times daily reached the target stimulation parameters in less time; however, there was no difference in clinical observations, clinical pathology, ECG, or gross necropsy findings when compared to the animals with stimulation increases four times daily. At the end of the titration period, stimulation intensity was manually increased further, and an expiratory reflex side effect was observed in all animals, indicating that the tolerance zone boundary remained present above the delivered stimulation intensity.
There were two adverse events during the study: A drug reaction to a sedative pre-medication, and an incident of Horner's Syndrome (drooping eyelid). Horner's Syndrome was deemed to be procedure-related, due to inadvertent excessive manipulation of the vagus nerve during implant, and subsequently resolved without treatment. There were no adverse events related to the device, automatic titration, or VNS therapy, and no mortality during the study. Lead impedance remained stable for the duration of the study in all animals.
All animals were successfully programmed with the TitrationAssist algorithm, which automatically performed the stimulation intensity increases as expected. Stimulation intensity of up to 2.5 mA was well tolerated by all animals. While the 4x/day stimulation increases reduced the duration of time needed to achieve target stimulation parameters, the more aggressive titration curve (which took approximately 30 days to reach target intensity) did not result in any side effects or adverse events. Unscheduled rollbacks to decrease the stimulation intensity were not needed during the study.
Device interrogations were performed at implantation procedures, weekly throughout the titration phase of the study, and prior to and following any stimulation intensity rollbacks. There were no unexpected or abnormal results with regard to the electrical performance of these devices and no abnormal clinical observations documented during interrogations. Lead impedance values remained within the normal range (1000-3000 Ω) in all animals.
At each scheduled magnet maneuver session, animals had three consecutive magnet activations (15-30 sec hold over PG) followed by device interrogation. In two animals, rollback was confirmed in three consecutive magnet activations. Two animals required fourth magnet activation, and two animals required fifth magnet activation. After the unsuccessful magnet activations were observed, it was determined that the staff was placing the magnet with an incorrect orientation; the appropriate orientation of the magnet over the device was subsequently used, and there were no further issues related to magnet use. All remaining magnet activations successfully reduced stimulation intensity. Appropriate magnet maneuver training will be a critical element of clinical implementation of this feature.
Weekly veterinary assessment did not reveal any abnormal respiratory rate, and there were no arrhythmias auscultated during these exams. Four of the animals gained weight during the study, and the remaining 2 animals had mild weight loss.
The vagus nerve with the implanted lead and the contralateral non-implanted vagus nerve were isolated and evaluated. Both vagus nerves were removed without incident and appeared normal in all animals.
Gross examination and collection of representative sections of all major organs (brain, heart, lung, liver, spleen, kidneys, and gastro-intestinal tract) was uneventful, and all tissue appeared clinically normal in all animals.
All six animals had blood count and serum chemistry values within normal limits at baseline and termination. Three animals had Gamma-Glutamyl Tranferase values below the reference range, and the other three animals had Globulin values below the reference range; all animals remained healthy and free of clinical symptoms and all these values were deemed clinically insignificant by the staff veterinarian.
Holter monitor recordings at baseline and end of study were unremarkable. Several animals had individual instances of ventricular escape beats, premature ventricular ectopic beats and junctional escape beats. None of the observations were considered by the reviewing cardiologist to be of clinical concern. All observations were consistent with normal canine physiology, and the cardiologist found no evidence that they were therapy-related. None of the Holter recordings contained any incidence of bradycardia, ventricular tachycardia, atrial fibrillation, atrial flutter, or ventricular fibrillation.
The VITARIA System delivers ART using VNS for the treatment of patients with heart failure using chronic VNS. ART using VNS has been shown to inhibit neural release of norepinephrine at cardiac effectors [8], restore autonomic balance (as reflected in improvements in HR variability and baroreflex sensitivity) [9,10], reduce systemic inflammation [11-13], increase coronary flow [14], exert antiapoptotic effects [9,15] and directly modulate reflex processing within peripheral ganglia of the cardiac nervous system [16]. In heart failure patients, VNS has been associated with improved heart rate, heart rate variability, left ventricular ejection fraction, six minute walk distance, NYHA class, and quality of life [5,6,17].
The most burdensome aspect of ARTis the adjustments of VNS needed for up-titration. In this study, a novel featurewas evaluated as an alternative to manual titration of VNS. VNS intensity increased automatically in small increments over a programmable time period, allowing stimulation to reach a programmable target of VNS intensity without the need for manual adjustments.
All animals that were enrolled in the study successfully completed the device implant phase and scheduled study duration. The TitrationAssist feature performed as expected, with all animals undergoing daily stimulation intensity increases and reaching the target amplitude of 2.5 mA. Lead impedance values remained in the normal range throughout the study. The tolerance zone boundary, at which the animals experience stimulation-related side effects, was present at the start and end of titration; however, the tolerance zone boundary was not encountered during titration due to the progressive and gradual increase in TitrationAssist stimulation intensity that elicited physiological adaptation.
There were no clinically relevant findings from the Holter ECG recordings, clinical pathology data, or gross necropsy observations for any animal that would suggest a detrimental effect on the animals due to the implanted device or chronic stimulation.
The VITARIA TitrationAssist feature performed as expected. All animals achieved the target stimulation parameters of 2.5 mA, pulse width of 250 μs, and frequency of 5 Hz without stimulation-related side effects or adverse events. TitrationAssist automatically and gradually increased stimulation intensity over the titration period without any further intervention, constituting a novel and promising technology for reducing the burden of titration in patients with implantable neurostimulation devices.