Migraine headaches are a common complaint described by patients and few medications have been designed solely for their treatment. Current knowledge of migraine pathogenesis relates to various neuropeptides, including calcitonin gene related peptide (CGRP), acting on blood vessels within the brain, causing vasodilation. Sensory fibers detect this change and perceive it as pain. This understanding has led to the development of CGRP monoclonal antibodies as a possible migraine treatment.
This class of migraine medication causes concern for possible cardiovascular side effects. Blocking CGRP and the vasodilatory process of migraines may pose a risk for exacerbating cardiovascular disease. CGRP has also demonstrated protective effects on the cardiovascular system by preventing against heart failure, deleterious cardiac remodeling, hypertension, and cell death. Additionally, patients with migraines are also believed to be at greater baseline risk for cardiovascular and cerebrovascular disease. Assessment of a cardiovascular risk profile is essential for the continued use of this medication class.
Various trials within phase II or III of study were analyzed for the risk profiles of CGRP monoclonal antibodies. At this time, no serious cardiovascular adverse effects have been found. The CGRP monoclonal antibodies did not increase rates of cardiovascular adverse events, when compared to placebo. The CGRP monoclonal antibodies were shown to be safe in patients with previous cardiovascular risk as well as those stressed to provoke an adverse cardiovascular event. Many of the phase II and phase III trials had significant female participation, representing a safe cardiovascular profile for those most commonly affected with migraines. This demonstrated that the medication class does not increase risk of cardiovascular side effects in its users.
Migraine, Calcitonin gene related peptide (CGRP), Monoclonal antibody, Headache, Cardiovascular
The pathogenesis of migraines remains an area of significant study today. Migraines are hypothesized to involve the trigeminal nerve and innervation of cranial vessels. Studies have demonstrated inflammatory particles likely trigger this cascade. This inflammation stimulates the neurons to depolarize and release calcitonin gene-related peptide (CGRP) [1]. CGRP then causes increased perfusion through cerebral vessels [2]. Dilation of these cerebral vessels stimulates sensory fibers of the trigeminal nerve, which is relayed to the brainstem as pain. The brain then amplifies the release of CGRP into cranial vessels, causing more vasodilation, inflammation, and leakage of vessels [1]. Further research has found that CGRP signaling may specifically occur through C-fibers and Aδ-fibers in the trigeminal ganglion. With the use of CASPR, a study has shown CGRP receptors are located at nodes of Ranvier in Aδ-fibers, while CGRP release occurs from C-fibers [3]. Blocking the effect of CGRP in cranial vessels was the proposed mechanism of calcitonin gene-related peptide monoclonal antibody for migraine treatment. Numerous studies have shown CGRP monoclonal antibodies to be an efficacious medication class [4-10]. Knowing the efficacy of the CGRP monoclonal antibodies, this review focuses on cardiovascular adverse events and possible safety profile expected for women.
Until recently, migraines have been treated with antihypertensive drugs, antidepressants, and antiepileptic medications [4]. These medications are associated with numerous side effects. Currently, one of the most efficacious treatments are triptans. Triptans act as agonists of serotonin receptors 5-HT1B and 5-HT1D and cause vasoconstriction of blood vessels [11]. This mechanism of vasoconstriction helps alleviate symptoms of a migraine and consequentially may trigger ischemic cardiac events. Therefore, many migraine medications present with cardiovascular side effects.
The importance of finding a safer and more effective migraine treatment led to the development of CGRP antagonists. Gepants were developed with the intention to inhibit one of the primary mechanisms behind the pathogenesis of migraines, CGRP. Although studies were initially encouraging, gepants, such as telcagepant, had low bioavailability and caused elevated transaminases. Research has changed focus to blocking CGRP ligand, or its receptor, with a monoclonal antibody [4].
Although CGRP antagonists are associated with significant side effects, the effects on the cardiovascular system have remained benign. Various studies have shown that antagonizing CGRP does not worsen myocardial ischemia, exacerbate stable angina, or cause other cardiovascular side effects [12-14]. An animal model of CGRP antagonism demonstrated no worsening of myocardial ischemia, following a decrease in coronary blood flow [12]. During treadmill exercise in patients with stable angina, CGRP antagonism caused no change in total exercise time, ST- segment depression, time to angina, or change in blood pressure [13]. Due to limited research on CGRP monoclonal antibodies, the lack of cardiovascular side effects associated with CGRP antagonists may represent promising safety outcomes associated with blockade of CGRP.
Two isoforms of CGRP allow for the efficacy of this class medication and may explain possible side effects. The first isoform is α-CGRP, which is most active in sensory neurons in the dorsal root ganglia. The second isoform, β-CGRP, can be found in the enteric nervous system. This is visualized in Figure 1 [4]. The efficacy and safety profile can be explained through the action of α-CGRP at the target organ. α-CGRP binds the CLR/RAMP1 receptor, acting on a G-protein Gαs cascade and Gαq/11 pathway. The impact of binding these receptors causes relaxation of vascular smooth muscle, vasodilation, cardiac contraction, and synaptic plasticity. The isoforms and their actions at various organ systems are explained in Figure 2 [15]. The vasodilation caused by α-CGRP is profound. In 1985, α-CGRP was found to be the most potent vasodilator in the human body [16]. The vasodilatory effects of CGRP are believed to be protective to the brain and heart in light of ischemic events.
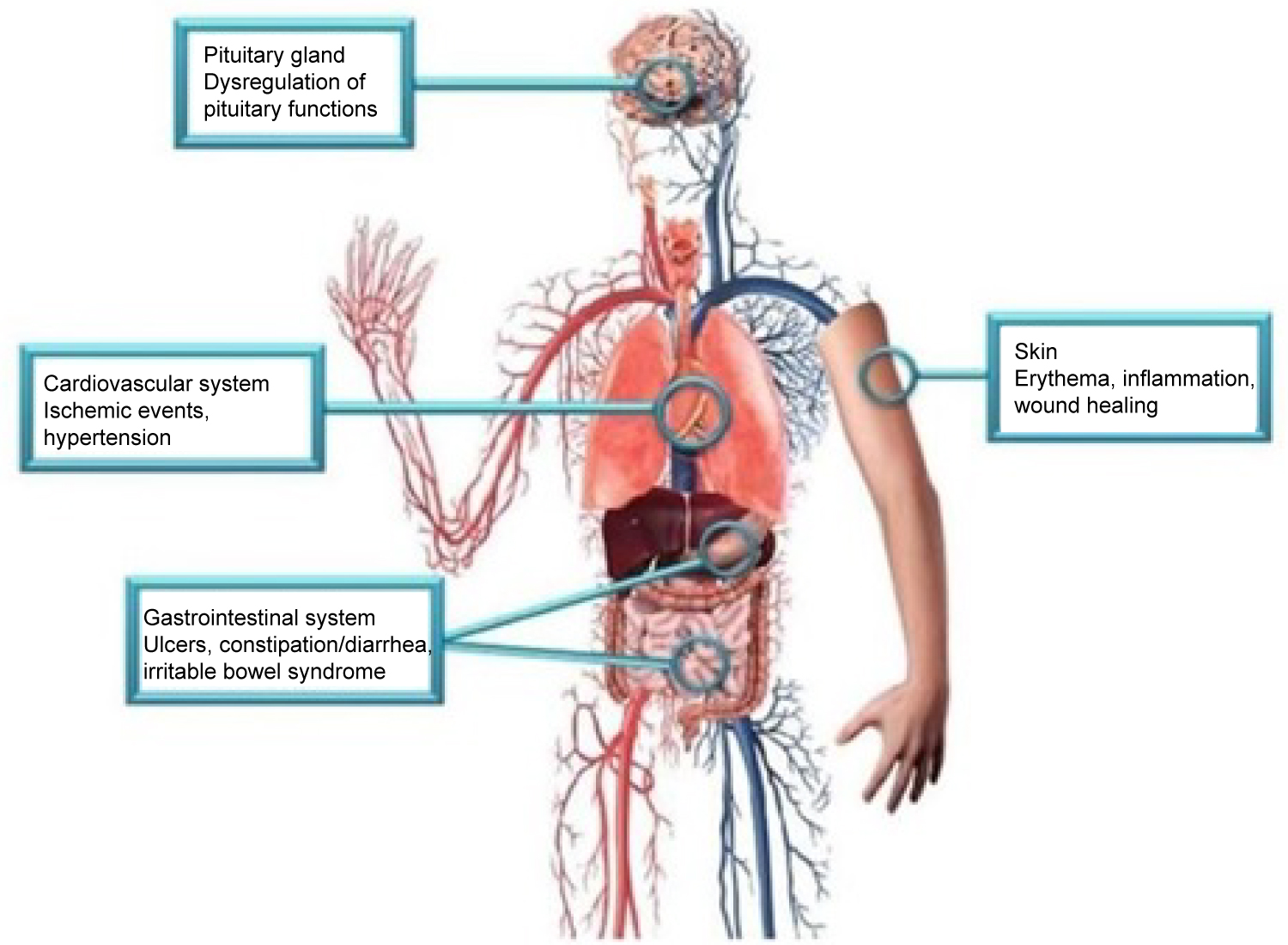 Figure 1: Side effects associated with CGRP blockade may be explained by the action of CGRP in the pituitary gland, cardiovascular system, gastrointestinal system, and skin [4].
View Figure 1
Figure 1: Side effects associated with CGRP blockade may be explained by the action of CGRP in the pituitary gland, cardiovascular system, gastrointestinal system, and skin [4].
View Figure 1
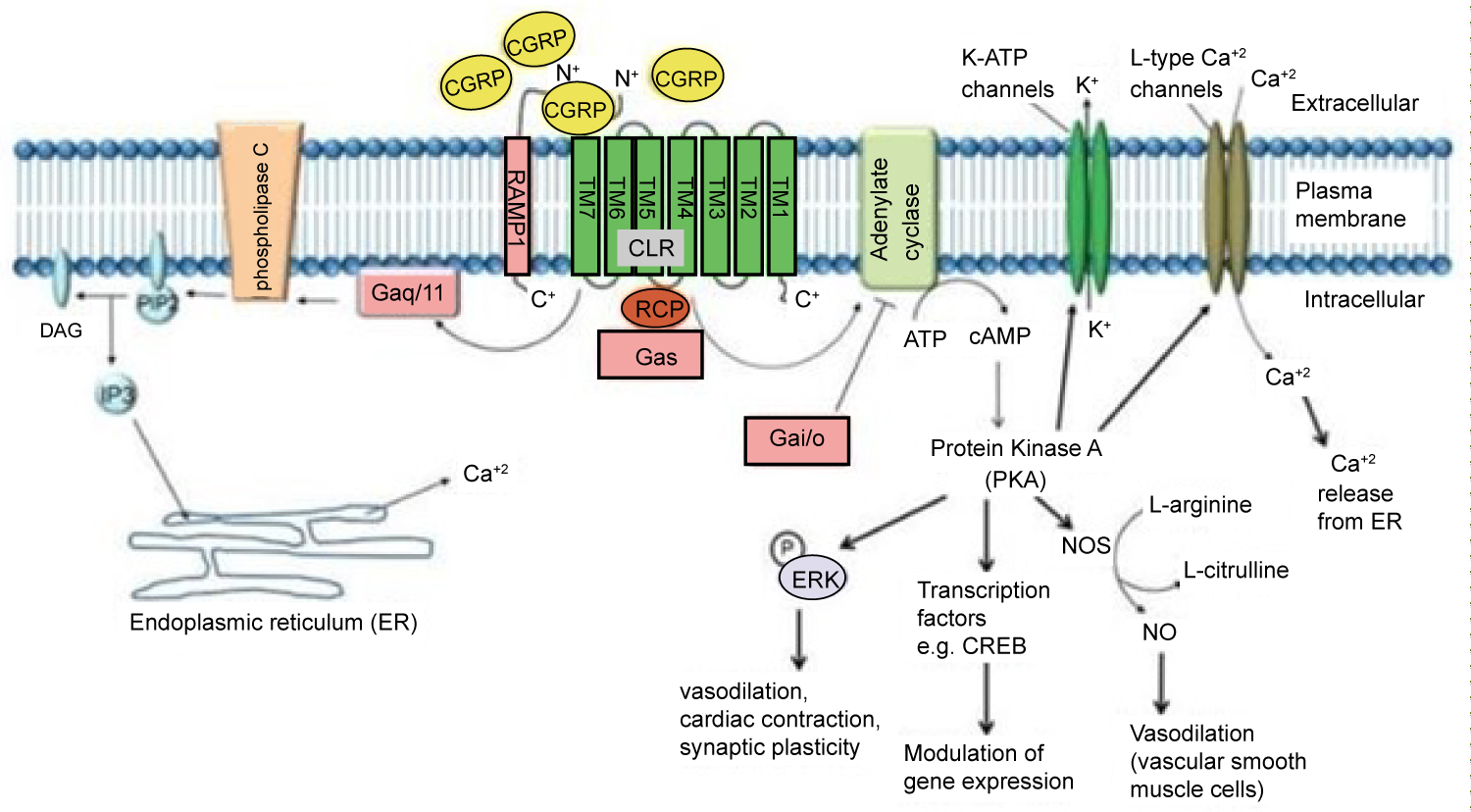 Figure 2: As a Gαs receptor, the binding of α-CGRP increases cAMP and protein kinase A levels. CGRP can also increase intracellular Ca+2 concentrations by acting through a Gαq/11 pathway [15].
View Figure 2
Figure 2: As a Gαs receptor, the binding of α-CGRP increases cAMP and protein kinase A levels. CGRP can also increase intracellular Ca+2 concentrations by acting through a Gαq/11 pathway [15].
View Figure 2
The relationship between CGRP blockade and cardiovascular risk may be explained through various molecular pathways. Specifically, this review identifies how CGRP protects against heart failure, ischemic heart disease, and hypertension. Numerous studies discussed below have been reviewed with this understanding in mind, to determine if adverse events frequently occurred.
Along with the vasodilatory effect, α-CGRP has been found to prevent cardiac myocyte apoptotic cell death and increase survival in heart failure. Under cellular stress, CGRP leads to decreased cell death through mechanisms such as increasing Bcl-2 and decreasing Bax expression, decreasing oxidative stress, and increasing nitric oxide production [17-19]. A comparison of heart failure in wild type and knock out CGRP mice found that WT mice had a greater survival rate at 28 days. The KO mice had increased incidence of deleterious cardiac remodeling presenting as left ventricular hypertrophy, cardiac fibrosis, apoptosis, necrosis, inflammation, and reduced angiogenesis [20]. CGRP protects against heart failure by blocking cell death and preventing adverse changes within the heart.
Similarly, there may be concern when using CGRP monoclonal antibodies in patients at risk for ischemic cardiac events [15]. Previous studies have demonstrated that CGRP prevented cellular death in mice exposed to 30 minutes of ischemia followed by 30 minutes of reperfusion. In the study, α-CGRP knockout mice had a reduction in heart function following the ischemic reperfusion insult. This was measured with creatine kinase, a marker of cellular death. At five, 15, and 30 minutes, the level of creatine kinase was markedly elevated in CGRP knockout mice when compared to wild type mice. Malonaldehyde (MDA) was used in the study to measure oxidative stress. Initially, both types of mice, KO and WT, had the same amount of MDA. After the ischemic refusion insult, the levels of MDA were significantly higher in CGRP knockout mice. The study concludes that CGRP helped to prevent oxidative stress and cellular death of the heart following ischemic reperfusion injury [21]. Based on this knowledge, patients with coronary artery disease are at high risk of adverse events when CGRP is blocked.
Studies have also demonstrated a connection between CGRP and hypertension. Hypertensive rats have been found to have decreased levels of CGRP. This suggests that higher levels of CGRP cause vasodilation, allowing for a decrease in blood pressure [22]. Blood pressure was compared in α-CGRP knockout mice and wild type mice. The knockout mice showed elevated systolic blood pressure and mean arterial pressure when compared to wild type mice [23]. α-CGRP may also impact hormonal cascades within the body that are important regulators of blood pressure. It is well known that the renin-angiotensin-aldosterone system plays a significant role in hypertension. α-CGRP decreases the ability of angiotensin II to increase blood pressure and cause vascular remodeling and oxidative stress [24]. CGRP has been shown to provide various protective effects in the cardiovascular system [15,24]. Knowing this, changes in vital signs reported during phase II and III trials will be a valuable measurement of assess how CGRP blockade affects the cardiovascular system.
The potential for this medication class to provoke an adverse cardiovascular event causes increased concern because the migraine patient population is already at higher baseline risk. Evidence supports a correlation between migraines and higher cardiovascular and cerebrovascular events [25]. A meta-analysis has shown patients with migraines are at higher risk of stroke and myocardial infarction, when compared to patients without migraines [26]. Phase II and III trials of CGRP monoclonal antibodies will provide an understanding of how patients with migraines are affected.
Various studies of CGRP monoclonal antibodies have assessed the safety profile of the medication class. Consistency in vital signs was found throughout the studies, as visualized in Figure 3 and Figure 4 [27-32]. Studies of patients with preexisting cardiovascular risk factors have also shown promise. No difference in adverse events were found between the drug treatment group and the placebo group [33]. Figure 5 and Figure 6 demonstrate no differences in cardiovascular measurements were found in patients with angina [29]. Common cardiovascular side effects that have been reported have been hypertension, tachycardia, palpitations, and increased heart rate [34]. These side effects were often explained by events independent from drug administration and previous medical problems [30,35,36]. Injection site reactions were consistently the most common adverse effect amongst all trial data. In addition, other common adverse effects included: Upper respiratory tract infection, sinusitis, nasopharyngitis, influenza, back pain, and fatigue [30,31,33-37]. The withdrawal rate from the studies and reasons for cessation were not significantly different between treatment and placebo groups [33-38]. These studies have demonstrated the protective vasodilatory effect of CGRP on the cardiovascular system, is not altered by the monoclonal antibodies.
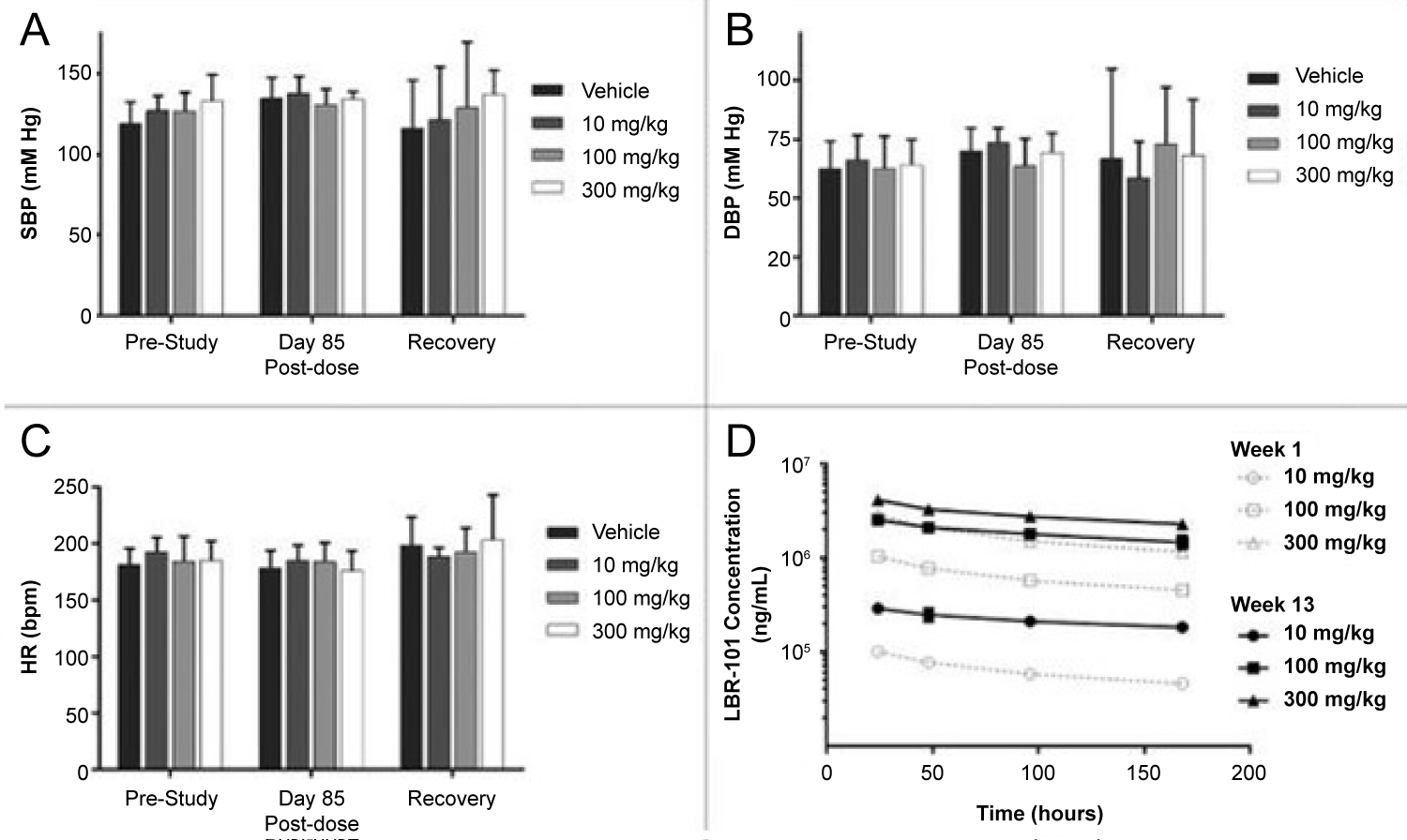 Figure 3: Data was collected prior to the first fremanezumab dose, at day 85 of dosing, and one week after dosing. Data collected during fremanezumab treatment demonstrated no significant difference in systolic blood pressure (A), diastolic blood pressure (B), and heart rate (C). Plasma concentrations were measured to assess accumulation of the drug with repeated dosing [27].
View Figure 3
Figure 3: Data was collected prior to the first fremanezumab dose, at day 85 of dosing, and one week after dosing. Data collected during fremanezumab treatment demonstrated no significant difference in systolic blood pressure (A), diastolic blood pressure (B), and heart rate (C). Plasma concentrations were measured to assess accumulation of the drug with repeated dosing [27].
View Figure 3
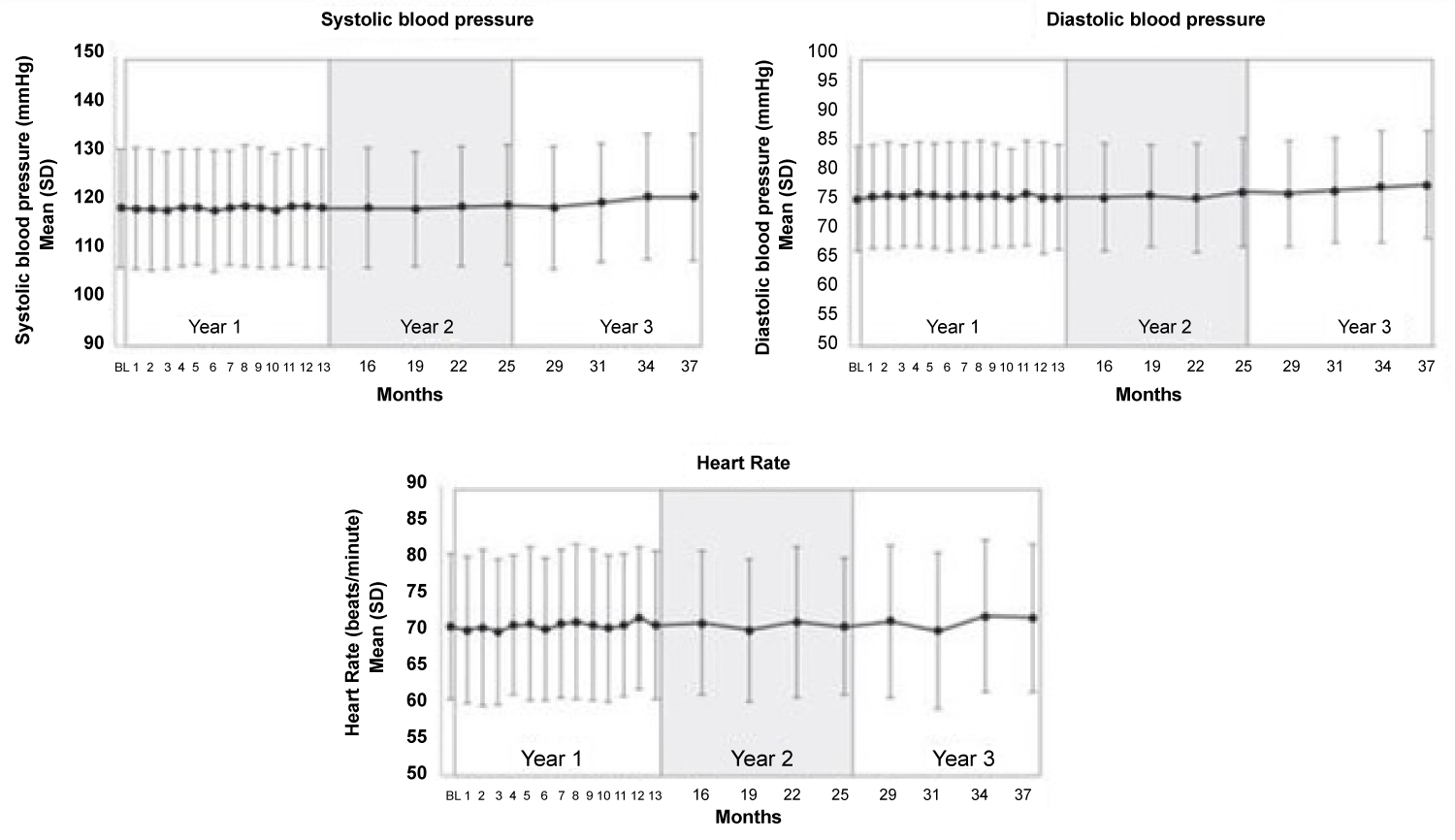 Figure 4: No significant changes in systolic blood pressure, diastolic blood pressure, and heart rate were found during 3.3 years of follow-up [30].
View Figure 4
Figure 4: No significant changes in systolic blood pressure, diastolic blood pressure, and heart rate were found during 3.3 years of follow-up [30].
View Figure 4
 Figure 5: Patients with chronic stable angina were treated with a monoclonal antibody and were assessed for cardiovascular changes during treadmill testing. There was also no difference in time to ≥ 1 mm ST-segment depression [29].
View Figure 5
Figure 5: Patients with chronic stable angina were treated with a monoclonal antibody and were assessed for cardiovascular changes during treadmill testing. There was also no difference in time to ≥ 1 mm ST-segment depression [29].
View Figure 5
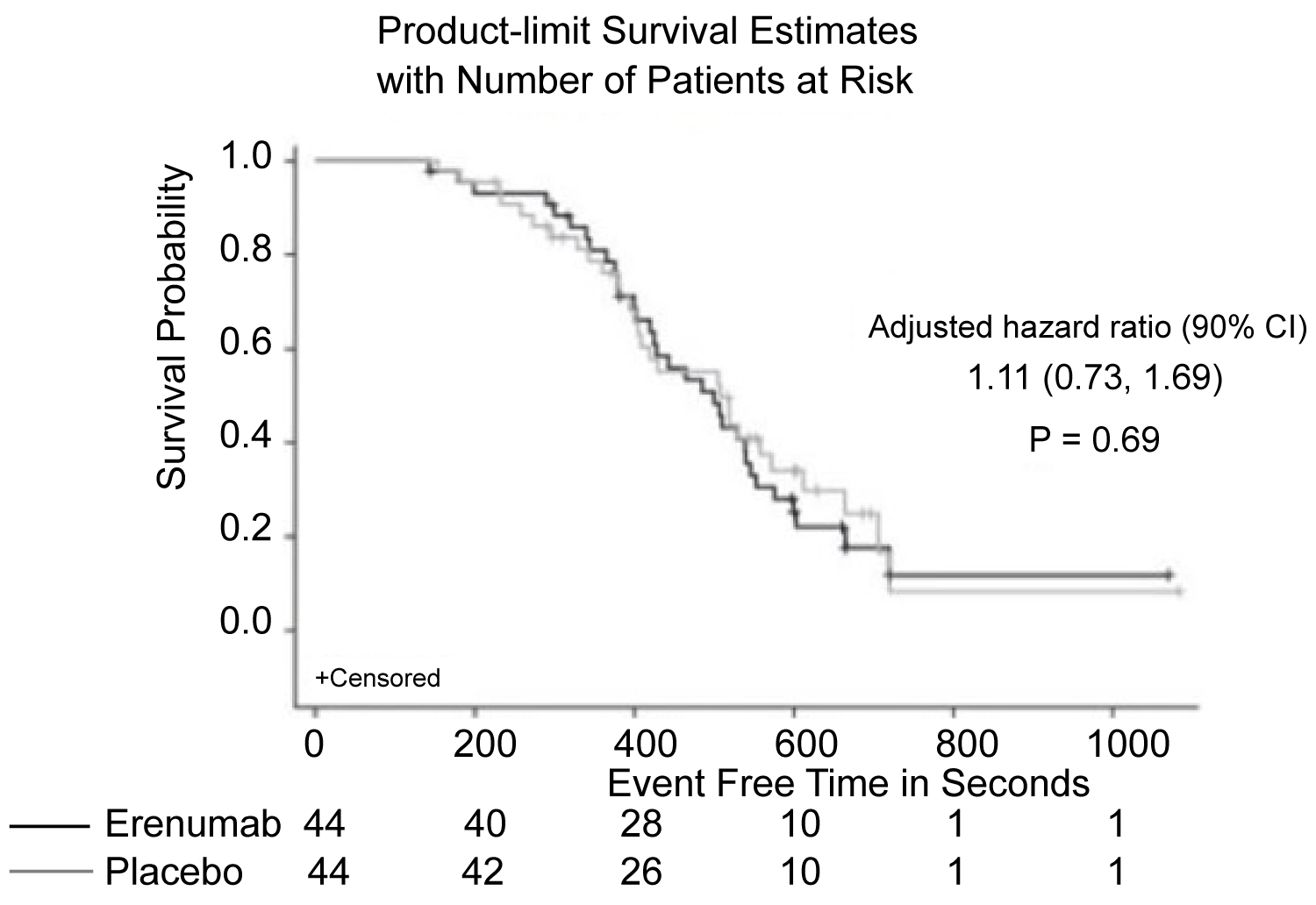 Figure 6: Patients with chronic stable angina also demonstrated no difference in time to exercise-induced angina, when comparing erenumab treatment and placebo [29].
View Figure 6
Figure 6: Patients with chronic stable angina also demonstrated no difference in time to exercise-induced angina, when comparing erenumab treatment and placebo [29].
View Figure 6
Many of the studies do not specifically examine how response to CGRP monoclonal antibodies varies based on gender. This is an important variable because migraine patients are more likely to be women [39]. The efficacy of the migraine treatment was not affected when tested in mostly female participants. Females comprised 85 percent of participants in a study that found a significant reduction in migraine days, when comparing the effects of Galcanezumab in both high and low frequency migraine patients [5]. Women may also have greater reliance upon the protective effects of CGRP when compared to males. Female sex steroids have been shown to modulate the vasodilatory effects of CGRP [40]. Therefore, studies that have high or entirely female participation represent research that is important to accurately assessing the safety profile of the target population. A study of 31 women compared placebo and Fremanezumab treatment and it found no significant difference in heart rate, blood pressure, and ECG parameters [28]. Research articles were analyzed for this specific population of interest. Numerous studies consisted of a high female participation. These studies demonstrated consistency in their lack of adverse cardiovascular outcomes [30-37]. Females consisted of anywhere from 77 to 87 percent of the populations studied in these trials. This may be extrapolated to represent a positive safety profile in the female population, following CGRP monoclonal antibody use.
Current studies have not demonstrated concern for cardiovascular side effects when blocking CGRP. A meta-analysis examined ten randomized, double-blind, placebo-controlled trials of the four CGRP monoclonal antibodies in adults, aged 16-65, with episodic migraine, to evaluate the safety and efficacy. When the CGRP monoclonal antibody treatments were compared to placebo, the study found no significant difference in withdrawal rates, withdrawal rates due to adverse effects, severe adverse effects, or any adverse effect. Table 1 lists the common adverse effects reported by all ten studies. As visualized in the table, none of the studies reported any cardiovascular side effects related to the treatment medication [41]. This study was a meta-analysis that included phase II and III trials, which are some of the most current data currently published on the CGRP monoclonal antibodies. The meta-analysis found no significant difference in the safety of the medications when compared to placebo. This demonstrates that current research supports the CGRP monoclonal antibodies as well-tolerated medications with few cardiovascular side effects.
Table 1: Adverse events of CGRP monoclonal antibodies compared to placebo. View Table 1
Understanding the cardiovascular risk profile of monoclonal antibodies for migraine is important for CGRP monoclonal antibodies to be consistently used in treatment. Previous descriptions of the side effect profiles of this medication class, have called for further long term assessment of adverse events. This review identifies the most recent phase II and III trials on the topic. Consistently, CGRP monoclonal antibodies have not shown a significant difference in adverse outcomes, cardiovascular or otherwise, when drug treatment groups are compared to placebo. Vital signs have not been significantly affected. Patients with prior cardiovascular conditions have also not shown a significant difference in outcome. Studies that demonstrate these results are important for the current understanding of the cardiovascular risk profile of CGRP monoclonal antibodies. Although patients with migraines are at an increased risk of cardiovascular and cerebrovascular disease, CGRP monoclonal antibodies have demonstrated efficacy with few safety concerns. Phase IV trials for CGRP monoclonal antibodies will continue to provide valuable information on the safety profile. CGRP monoclonal antibodies require further study in patients with major cardiovascular risk. Currently, no research has shown cardiovascular risk with the use of CGRP monoclonal antibodies but a further exploration into their long term viability is warranted.
I would like to acknowledge Deen M, Correnti E, Kamm K, Kelderman T, Papetti L, Rubio-Beltrán E, Vigneri S, Edvinsson L, Maassen Van Den Brink A and Springer Nature for the use of Figure 1 within the contents of this manuscript. No changes were made to this figure. A link to the Creative Commons license is: http://creativecommons.org/licenses/by/4.0/.
Kumar A, Potts JD, DiPette DJ and Frontiers Publishing for the use of Figure 2 in text
Walter S, Alibhoy A, Escandon R, Bigal ME and Taylor and Francis Online for the use of Figure 3 in text. The Journal’s website: www.tandfonline.com.
Ashina M, Goadsby PJ, Reuter U, Silberstein S, Dodick D, Rippon GA, Klatt J, Xue F, Chia V, Zhang F, Cheng S, Mikol DD and Sage Publishing for the use of Figure 4 in text.
Depre C, Antalik L, Starling A, Koren M, Eisele O, Lenz RA, Mikol DD and Wiley Online Library for the use of Figure 5 and Figure 6 in text.
Xu, D., Chen, D., Zhu, L., Tan, G., Wang, H., Zhang, Y., & Liu, L and Sage Publishing for the use of Table 1 in text.
There were no sources of financial support.
Each author contributed equally to the manuscript.