The use of marihuana is becoming more frequent in young people, the association between psychosis and cannabis has been described, especially in young people, we present a clinical case of psychosis associated with cannabis in a young man with normal Brain Magnetic resonance, but abnormalities in tractography mainly in the connectivity between cuadate nucleus and frontal lobe.
Psychosis, Cannabis, Caudate nucleus, Frontal lobe, Tractography
Several longitudinal population studies strongly association between cannabis and psychosis, particularly in individuals who begin consumption before adulthood [1].
The Cannabis is the most common used drug among young people, the rates recorded around of the onset of psychosis as between 35-45%, the continued use of marihuana following onset of psychosis is associated with poorer outcomes, delays in remission, suicidal behaviour, violence [2].
In prospective investigations in first-episode psychosis cannabis users an Australian study report a 51% relapsed rate over 15 months follow-up among users compared with 17% among non-users [3]. Similarly, a Dutch study reported a 42% relapsed rate among persistent cannabis users compared with 17% among who never used [4].
The risk of developing a psychotic episodes also is increased in regular cannabis use a dose dose-dependent manner, in studies between users and non users increase risk 1.4 fold, and heavy use ( daily users) increases risk 2.1 times that non-users [5].
Despite the reporting of earlier age at psychosis onset in cannabis users, the exact reason for this clinical condition has not yet been established, but an increase in cannabinoids CB1 receptors density in prefrontal áre as during adolescence has been proposed to be responsible in vulnerable patients [6].
Acute dopamine release by measuring changes in D2/3 binding in specific radioligands and following up the extracellular dopamine levels reported in a number of cases suggested a 20% reduction of dopamine at striatal level at the D2/3 receptor in A subject exposed to cannabis [7].
Neuroimaging studies have found that prolonged use of cannabis could be responsible for a decrease in brain volume due to loss of inhibitory neurons especially in the neocortex area involved in psychotic symptoms there is a lack of a normal gabaergic neural mechanism in the cortex Anterior cingulate (CCA), dorsolateral (CPF), and primary motor and visual cortex. It is thought that such an inhibitory mechanism is essential for normal cognitive functioning and therefore its deficit would lead to the perceived cognitive dysfunction in schizophrenia [8,9]. We present a clinical case with psychosis in cannabis user and abnormlities in tractography.
A 19-years-old man, is hospitalized due to visual and auditory hallucinations with mandatory orders associated with motor restlessness following smoking marijuana.
Smoker of marijuana onset 14-years-old. In the neurological and psychiatric exam is observed with commitment in sensoperception thought, glabellar and hoffman's signs. The lab test was normal MRI and tracotography were done (Figure 1 and Figure 2).
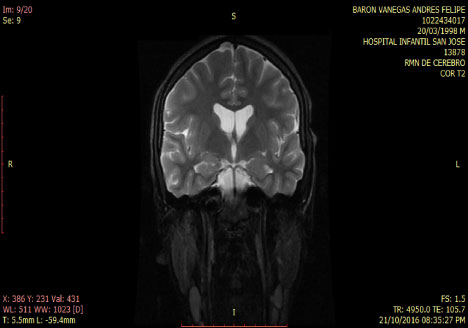 Figure 1: T2 Coronal MRI: normal. View Figure 1
Figure 1: T2 Coronal MRI: normal. View Figure 1
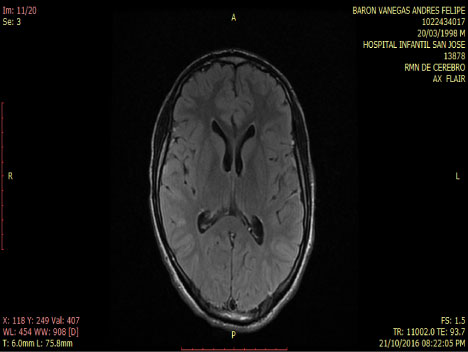 Figure 2: Axial - Flair MRI: Normal. View Figure 2
Figure 2: Axial - Flair MRI: Normal. View Figure 2
Since the endogenous cannabinoid system has been described, it is known that the psychoactive effects associated with the use of marijuana are because the pharmacological interaction of Δ9-tetrahydrocannabinol present in cannabis preparations with one of the molecular elements of this system, CB1 cannabinoid receptors.
Three types of cannabinoid receptors CB1, CB2Y and CB3 have been described so far.
The CB1 receptor (rCB1), also called the central receptor, is mainly distributed in the frontal region of the cerebral cortex, the basal ganglia, the cerebellum, the limbic system and the hypothalamus at the level of the central nervous system [10]. Are mainly confined to presynaptic terminals where they modulate the release of excitatory and inhibitory neurotransmitters; Generally promoting the inhibition of their reléase.
These receptors at the glutamatergic terminals fulfill an excitatory agonist neuroprotective function and responsible for the effects of THC on locomotion, hypothermia, analgesia and catalepsy.
On the other hand, the CB1s expressed in GABA ergic terminals are critical for the memory deficits promoted by the THC as well as for stress and reward mechanisms [11].
In people with schizophrenia regardless of whether or not they have been consuming cannabis an increase of rCB1 appears in area 9 of brodmann, whereas in cannabis users unrelated to the psychiatric diagnosis the increase of rCB1 is located in the area of the nucleus Caudate and put a men which are structures of the striatum [12] (Figure 3 and Figure 4).
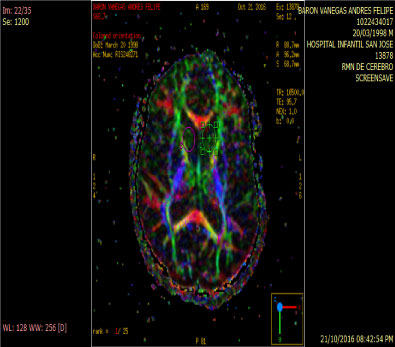 Figure 3: Anitropic Map: ROI Caudate Nucleus. View Figure 3
Figure 3: Anitropic Map: ROI Caudate Nucleus. View Figure 3
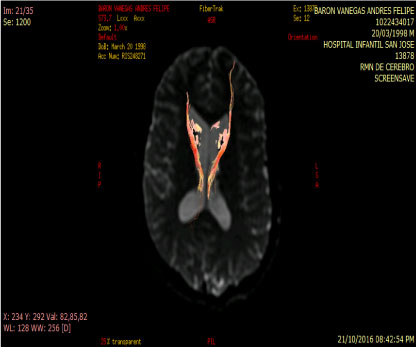 Figure 4: Tractography: Abnormal by loss of connectivity between the cuadate nucleus and frontal lobe. View Figure 4
Figure 4: Tractography: Abnormal by loss of connectivity between the cuadate nucleus and frontal lobe. View Figure 4
On the other hand, the administration of THC leads to an increase in the concentration of dopamine in the ventral tegmental area and in the nucleus accumbens (NAc) that is involved in the mesolimbic-mesocortical pathway responsible for the addiction to psychoactive substances, probably due to inhibition of the neurotransmission of γ-aminobutyric acid (GABA) so that a positive reinforcement is created. In addition, repeated long-term administration may produce the phenomenon of hypofrontality, leading to the establishment of toxic habit by reduction of dopamine in the prefrontal cortex (CPF). The projective symptoms that characterize hypofrontality refer in this context to impulsivity, persistence and repetition behavior and lack of extinction of behavior. In the case of impulsivity in particular, these are problems in delaying rewards, something that would explain the uncontrolled repetition of drug administration despite the circumstances and consequences, as observed in the abuse [12].
An abnormality in the neurotransmission of dopamine primarily in the striatal region is considered to be the final pathway that accounts for some of the prominent psychotic symptoms.
The acute effects of cannabinoid agonists are probably modulated by genetic factors. Several studies have explored the influence of genes mostly involved in schizophrenia and acute effects of cannabinoid psychosis:
COMT: Catechol-o-Metyltransferase enzyme plays an important role in the decomposition of dopamine, a mutation in the COMT V gene (108/158) leads to a significant reduction in the activity of this enzyme, patients who have COMT polymorphisms have a Moderate risk of developing psychotic symptoms.
DAT1: In the striatum the synaptic dopamine clearance is highly dependent on the dopamine transporter, a polymorphism of the DAT1 gene is associated with low transport and high levels of dopamine in the striatum. Bhattacharyya, et al. Showed that individuals with one allele with 9 replicates showed greater sensitivity to presenting THC-induced psychotic symptoms.
AKT1: Inactivates glycogen synthase kinase by phosphorylation and this interaction has been implicated with schizophrenia. Cannabinoids stimulate the AKT1 pathway through CB1 receptors. Bhattacharyya and colleagues found that a polymorphism in the AKT1 gene (the GG genotype of a single nucleotide polymorphism rs1130233) induces moderate sensitivity to acute psychotic episodes by THC.
BNF: Factor Literature reports have found that patients who suffer polymorphisms in these genes associated with an early age in cannabis use are more susceptible to having a psychotic episode [1].
Studies suggest a variety of genetic, environmental, and early cannabinoid use as factors predisposing to a psychotic syndrome [13].