Fever is one of the leading signs of the inflammatory process and it is one of the mechanisms that activate the immune system to defend the organism from various pathogens. For this goal, the peripheral blood mononuclear cells (PBMC) are among the first to be mobilized by triggering their capacity for phagocytosis and inflammatory cytokine production. However, their activation in the living organism is the outcome of several factors, including fever. The aim of the present work was to examine the effect of elevated temperature only on the capacity of human PBMC to produce inflammatory cytokines.
Stimulated and non-stimulated PBMC from healthy donors were treated with 37°C and 40°C for 4 and 24 hours. At the end of the incubation period the level of TNFα, IL-1β, IL-6, IL-10, IL-1ra, IL-2 and IFNγ was detected using ELISA kits.
Non-stimulated PBMC incubated at 37°C produced similar amounts of TNFα and IL-1β at 4 and 24 hrs. The expression of IL-6, IL-10 and IL-1ra was higher after 24 hrs as compared with that produced after 4 hrs. As for LPS-stimulated cells the production of all cytokines tested was increased significantly during 24 hrs of incubation except for the level of TNFα that did not differ significantly between 4 and 24 hrs of stimulation. Non-stimulated PBMC did not secrete detectable amounts of IL-2 or IFNγ, and that produced by PMA/ionomycin stimulated cells was significantly higher after 24 hrs of incubation as compared to 4 hrs. Elevation of the incubation temperature from 37°C to 40°C caused various degrees of inhibition in the generation of both pro- and anti-inflammatory cytokines.
Exposure of unstimulated and stimulated PBMC to 40°C induced various degrees of inhibition in the production of both pro- and anti-inflammatory cytokines compared to cells incubated at 37°C. This observation may clarify the way the immune cells react in cases with fever.
Temperature, Fever, Immunity, Mononuclear cells, Cytokines, Inflammation
Since centuries ago elevated body temperature has been designated as one of the four signs of inflammation. However, even in now days at the course of everyday practice, existence of temperature fluctuations is one of the first questions the patient is asked for. Increased temperature reflects the occurrence of a number of complex mechanisms including modulation of immune responses that play a major role in the defense against pathogens and other intruders. In this sense mobilization and increased number of peripheral immune cells capable to produce cytokines is imperative for abating the inflammatory process. In a previous study from our laboratory we have shown that isolated human peripheral blood mononuclear cells (PBMC) exposed to 40oC showed an increase in both phagocytic ability and augmented phagocytic index [1]. However, an additional function of the immune cells, i.e. their capacity for cytokine production at elevated temperature merits attention. Lundeland et al. [2] have shown that both hypo- and hyperthermia exert a modulatory effect on whole blood cytokine release. While TNFα production increased during hyperthermia of 40°C that of IL-1β was decreased. Hypothermia of 33°C did not affect TNFα production, but caused an increase in IL-1β release. The generation of IL-10 was unaffected by both hypo- and hyperthermia. Although TNFα and IL-6 are both pro-inflammatory cytokines, it appears that elevated temperature acts differently on their expression. Thus, Ensor et al. [3] have reported that LPS stimulated human macrophages exposed to 40°C for 18 hours did not show difference in IL-6 production compared to that obtained at 37°C. On the other hand, the expression of TNFα was completely inhibited at 40oC compared with the results at 37°C. It is notable that cells, other than immunocytes, may alter their capacity for cytokine production when exposed to elevated temperatures. Thus, hyperthermia combined with glycolipids enhanced the production of pro-inflammatory cytokine release by murine ovarian cancer cell lines [4].
Variations in cytokine productions by PBMC with fever duration has been reported in infectious diseases, such as measles [5], fever with thrombocytopenia syndrome [6], Crimean-Congo hemorrhagic fever [7], as well as in cases with auto-inflammatory syndromes represented by periodic fever [8,9]. However in those cases, the effect of factors other than elevated temperature on cytokine production by PBMC cannot be excluded. Therefore, the aim of the present work was to examine cytokine production by isolated human PBMC at high temperature, i.e. 40°C compared to that at 37°C.
The study was approved by the Rabin Medical Center Institutional Review Board. We did not study humans, but used blood cells which were purchased from the local blood bank. Hence the Review Board waived the need for informed consent.
PBMC were separated from adult blood bank donors' venous blood by gradient centrifugation using Lymphoprep-1077 (Axis-Shield PoC AS, Oslo, Norway). The cells were washed twice in phosphate buffered saline (PBS) and suspended in RPMI-1640 medium (Biological Industries, Beith Haemek, Israel) containing 1% penicillin, streptomycin and nystatin and supplemented with 10% heat inactivated fetal calf serum (designated as complete medium CM).
Peripheral blood mononuclear cells incubated without LPS or PMA were designated as non-activated cells. 2x106/ml of PBMC suspended in CM were incubated for 4 or 24 hrs without or with 50ng/ml lipopolysaccharide (LPS, E. coli, Sigma) to determine the secretion of TNFα, IL-1β, IL-6, IL-1ra and IL-10, or with 1μg/ml of phorbol meristate acetate (PMA-Sigma, Israel) and 0.5μg/ml of ionomycin (Sigma, Israel) to evaluate IL-2 and IFNγ production.
The tubes were incubated at 37°C or 40°C in a water bath. At the end of the incubation period, the cells were removed by centrifugation and the supernatants were kept at -70°C until assayed for cytokine content.
The concentration of cytokines in the supernatants was tested using ELISA kits specific for human cytokines (Biosource International, Camarillo, CA) as detailed in the manufacturer's guide-line. Each kit is specific for one individual cytokine. The detection level of all cytokines was 30pg/ml. The percentage of the CV of the ELISA assay for the cytokines examined was 2% to the mean or less.
Data was analyzed using two tailed paired Student's t-test to compare the effect of temperature on cytokine production at 37°C vs. 40°C and independent t-test to compare the findings at 4 and 24 hrs. The results are expressed as mean ± SEM. P value < 0.05 was considered as statistically significant.
The spontaneous or LPS-induced production of TNFα by PBMC incubated for 4 hrs as compared to 24 hrs did not differ significantly either at 37°C (p=0.22, p=0.44, respectively) or at 40°C (p=0.38, p=0.79, respectively). However, the spontaneous secretion of TNFα at 40°C was significant lower by 35% at 24 hrs of incubation (p=0.0025) whereas LPS-induced secretion of TNFα was reduced by 62% and by 54% at both 4 hrs and at 24 hrs of incubation, respectively (p< 0.001) as compared to PBMC incubated at 37°C (Figure 1).
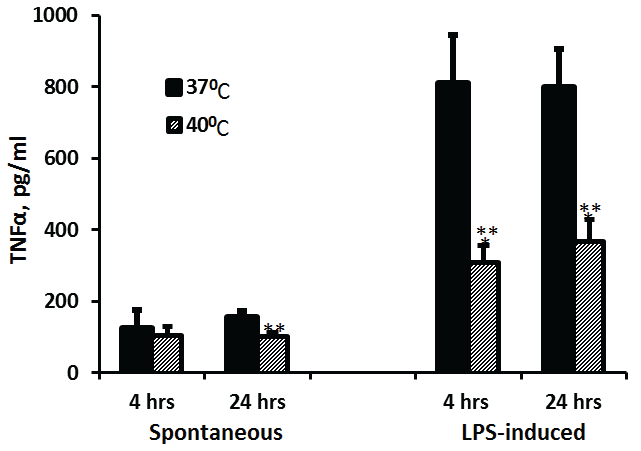 Figure 1: Effect of temperature on TNFα production
Figure 1: Effect of temperature on TNFα production
2x106 PBMC were incubated for 4 hrs or 24 hrs without or with LPS. The level of IL-1β in the supernatants was tested by ELISA. Each column represents the mean of 12-16 experiments. Bars represent SEM. Asterisks represent statistically significant difference between PBMC incubated at 37°C and 40°C (**p< 0.01; ***p< 0.001).
View Figure 1
The spontaneous secretion of IL-1β was similar at 4 and 24 hrs of incubation and was not affected by the temperature (p=0.57 and p=0.73 for 37°C and 40°C respectively). At 37°C, IL-1β production induced by LPS was higher by 64% after 24 hrs of incubation as compared with that after 4 hrs (p=0.009). LPS-induced secretion of IL-1β did not differ significantly between PBMC incubated at 40°C for 4 or 24 hrs (p=0.286). However, the production of IL-1β by LPS-stimulated cells incubated at 40°C was 45% and 63% lower as compared with cells incubated at 37°C for 4 hrs or 24 hrs respectively (p< 0.001) (Figure 2).
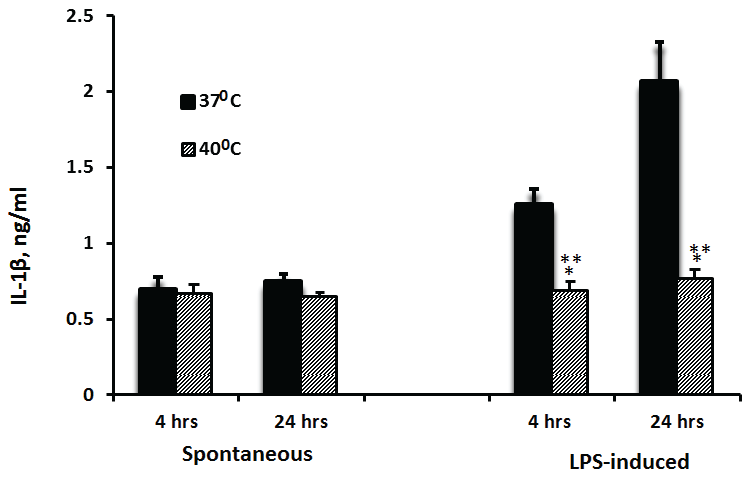 Figure 2: Effect of temperature on IL-1β production
Figure 2: Effect of temperature on IL-1β production
2x106 PBMC were incubated for 4 hrs or 24 hrs without or with LPS. The level of IL-1β in the supernatants was tested by ELISA. Each column represents the mean of 12-16 experiments. Bars represent SEM. Asterisks represent statistically significant difference between PBMC incubated at 37°C and 40°C (**p < 0.01; ***p < 0.001).
View Figure 2
While non-stimulated PBMC produced 64% more IL-6 when incubated at 37°C for 24 hrs as compared with 4 hrs (p=0.09), the secretion of IL-6 by cells incubated at 40°C did not differ significantly between 4 and 24 hrs of incubation. However, the production of IL-6 by non-stimulated PBMC incubated at 40°C was lower by 33% at 4 hrs (p=0.08) and 62% at 24 hrs (p< 0.001) of incubation as compared to cells incubated at 37°C. LPS-stimulated PBMC, incubated for 24 hrs at 37°C or at 40°C produced 38% and 70% more IL-6 than cells incubated for 4 hrs at the same temperatures (p=0.066, p=0.018, respectively). LPS-induced production of IL-6 was significantly reduced by 57.5% and 41% at 4 and 24 hrs of incubation, respectively (p< 0.001) when PBMC were incubated at 40°C as compared with 37°C (Figure 3).
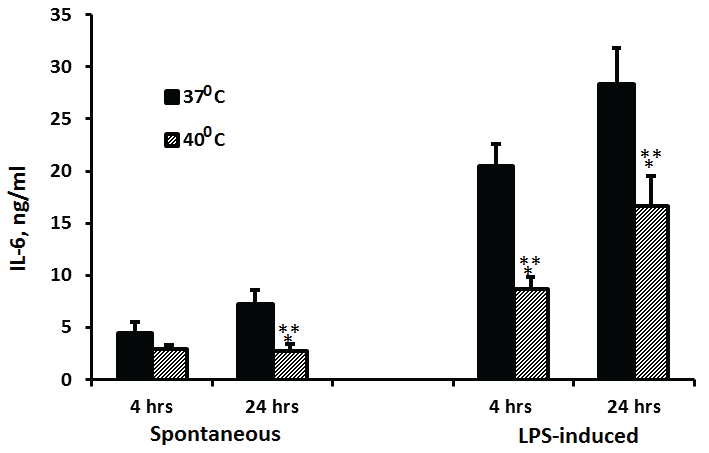 Figure 3: Effect of temperature on IL-6 production
Figure 3: Effect of temperature on IL-6 production
2x106 PBMC were incubated for 4 hrs or 24 hrs without or with LPS. The level of IL-6 in the supernatants was tested by ELISA. Each column represents the mean of 12-16 experiments. Bars represent SEM. Asterisks represent statistically significant difference between PBMC incubated at 37°C and 40°C (***p < 0.001).
View Figure 3
Non-stimulated PBMC did not produce detectable amounts of either IL-2 or IFNγ. The production of IL-2 or IFNγ at 37°C by PMA/ionomycin- stimulated PBMC was 71% and 77% higher at 24 hrs of incubation as compared with 4hrs (p< 0.001 and p=0.03, respectively). The secretion of both cytokines by cells incubated at 40°C did not differ significantly between 4 and 24 hrs of incubation (p=0.4). PBMC incubated for 24 hrs at 40°C produced 58% and 65% less IL-2 or IFNγ, respectively, as compared to cells incubated at 37°C (p< 0.001), whereas after 4 hrs of incubation, the secretion of these cytokines by cells incubated at both temperatures was similar (p>0.1) (Figure 4).
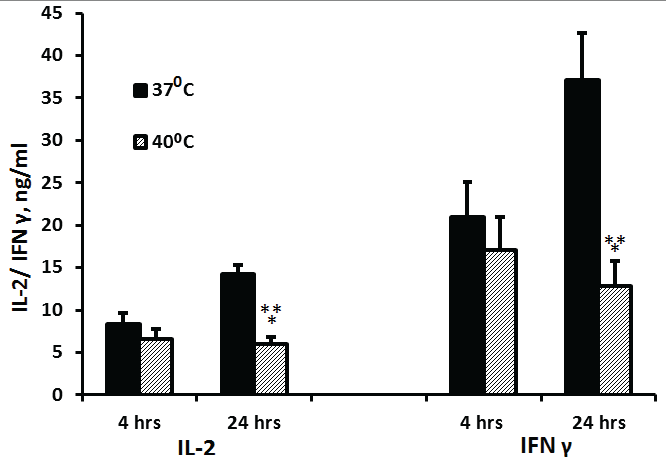 Figure 4: Effect of temperature on IL-2 and IFNγ production
Figure 4: Effect of temperature on IL-2 and IFNγ production
2x106 PBMC were incubated for 4 hrs or 24 hrs with PMA and ionomycin. The level of IL-2 or IFNγ in the supernatants was tested by ELISA. Each column represents the mean 16 experiments. Bars represent SEM. Asterisks represent statistically significant difference between PBMC incubated at 37°C and 40°C (***p< 0.001).
View Figure 4
The secretion of IL-10 at 37°C by non-stimulated PBMC was 30% higher after 24 hrs of incubation as compared to 4 hrs (p=0.038) and was similar at both time periods when the cells were incubated at 40°C. LPS-stimulated PBMC incubated for 24 hrs at 37°C or 40°C produced x4.5 or 79% more IL-10 as compared with cells incubated for 4 hrs at similar temperatures, respectively (p< 0.001). The production of IL-10 by LPS-stimulated PBMC incubated at 40°C was inhibited by 41% (p=0.0014) and 77% (p< 0.001) at 4 and 24 hrs of incubation, respectively, as compared to cells incubated at 37°C and reduced by 37% (p< 0.001) when non stimulated PBMC were incubated for 24 hrs at both temperatures (Figure 5).
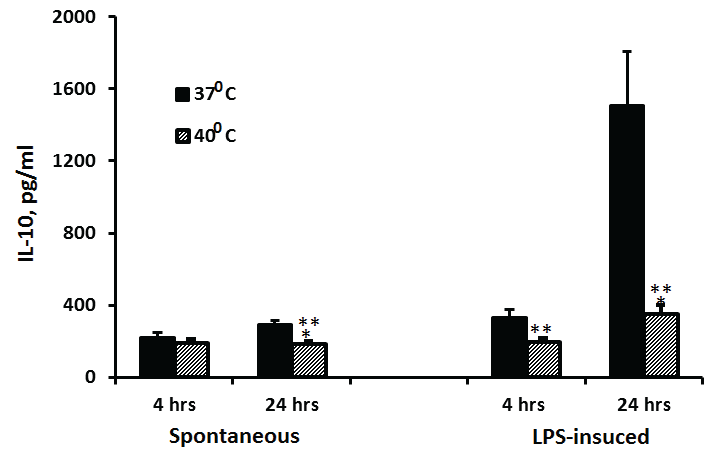 Figure 5: Effect of temperature on IL-10 production
Figure 5: Effect of temperature on IL-10 production
2x106 PBMC were incubated for 4 hrs or 24 hrs without or with LPS. The level of IL-10 in the supernatants was tested by ELISA. Each column represents the mean of 12-16 experiments. Bars represent SEM. Asterisks represent statistically significant difference between PBMC incubated at 37°C and 40°C (**p< 0.01; ***p< 0.001).
View Figure 5
The production of IL-1ra by non-stimulated PBMC was 3.25 times (p< 0.001) and 68% (p< 0.01) higher at 24 hrs by cells incubated at either 37°C or 40°C, respectively, as compared with cells incubated for 4 hrs at the same temperatures. However, the secretion of IL-1ra by non-stimulated PBMC incubated for 4 hrs and 24 hrs at 40°C was lower by 58% and 73%, respectively as compared with cells incubated at 37% (p< 0.001), and that by LPS-stimulated PBMC was reduced by 55% (p=0.012) and 71% (p< 0.001) respectively (Figure 6).
 Figure 6: Effect of temperature on IL-1ra production
Figure 6: Effect of temperature on IL-1ra production
2x106 PBMC were incubated for 4 hrs or 24 hrs without or with LPS. The level of IL-1ra in the supernatants was tested by ELISA. Each column represents the mean of 12-16 experiments. Bars represent SEM. Asterisks represent statistically significant difference between PBMC incubated at 37°C and 40°C (*p< 0.05; ***p< 0.001.
View Figure 6
The results of the study demonstrate that the capacity for cytokine production by isolated human PBMC depends on their stimulation, incubation period and temperature's degree. While PBMC non-stimulated by LPS or PMA and ionomycin, incubated at 37°C produced similar amounts of TNFα and IL-1β following 4 and 24 hrs of incubation, the secretion of IL-6, IL-10 and IL-1ra was higher after 24 hrs compared to that produced after 4 hrs. As for LPS-stimulated cells, only the secretion of TNFα did not differ significantly between 4 and 24 hrs of stimulation, the production of all other cytokines tested was increased significantly after 24 hrs of incubation. Non-stimulated PBMC did not secrete detectable amounts of IL-2 or IFNγ, whereas the level of these cytokines produced by PMA/ionomycin stimulated cells was significantly higher after 24 hrs of incubation as compared to 4 hrs. Elevation of the incubation temperature from 37°C to 40°C caused various degrees of inhibition in the generation of both pro- and anti-inflammatory cytokines. It has been reported that pretreatment of human macrophages with a temperature of 39.5°C enhanced LPS induced TNFα and IL-6 production [10]. However, activation of the immune cells is only a part of a number of immune modulatory effects that participate in the healing process. Cao et al. [5] have reported a decreased IFN-α and IFN-β mRNA expression in children with measles compared to controls. On the other hand, the anti-inflammatory cytokine IL-10 was significantly elevated. It seems that various pathogens act differently on the cellular immune response. Thus, it was reported that PBMC of patients with typhoid fever produce a significantly increased amount of a number of cytokines such as IFN-γ, TNF-β, IL-9, and IL-13 [11], suggesting a predominance in the activation of Th1 or Th2 cells by the particular pathogen inflicting the disease. Alterations of the cytokine profile have been observed in conditions accompanied by fever and inflammation others than those caused by pathogens. Compared to controls, stimulated and non-stimulated PBMC from patients with familial Mediterranean fever showed increased secretion of IL-6 and TNFα during crisis, whereas that of IL-1β and IL-1α was increased only by LPS stimulated cells. Non-stimulated PBMC showed the lowest yield of IFN-γ and IL-4 [9]. During the febrile episodes of an autoinflammatory process characterized by periodic fever, aphthous stomatitis, pharyngitis and cervical adenopathy, the peripheral blood concentrations of IL-6, IL-8 and IL-1β were elevated, whereas those of IL-2, IL-4, IL-5 and IL-10 were insignificant [8]. Studies with rats have shown that intra-peritoneal injection of zymosan induced a febrile response with an increased expression of IL-1β, IL-6 and TNFα within the central nervous system of the animals [12]. It is noticeable that treatment of ovarian cancer in mice by applying intra-peritoneal hyperthermia of 43°C for 10 min. did not trigger secretion of pro-inflammatory cytokines. Their enhanced expression was achieved only after combination of hyperthermia with α-galactosylceramide [4]. The mechanism by which hyperthermia stimulates immune cells for cytokine production is intricate. This subject has been thoroughly reviewed by Hasday et al. [13]. The authors imply that fever activates a heat shock factor-1 serving as a trigger for development of heat shock response with subsequent production of inflammatory cytokines by immune cells. Heat shock proteins are produced intracellular, but under stress conditions they are unconstrained from the cells and initiate the production of inflammatory cytokines [14]. It is plausible that hyperthermia with subsequent release of heat shock proteins underlies the mechanism by which tumor cells release cytokines with anti-tumor activity [15]. Regarding the close relationship between toll-like receptors (TLRs) and the immune- as well as the adaptive immune responses it is conceivable that elevated temperature may modulate immune function via TLRs' activation. The enhanced expression of the pro-inflammatory cytokines TNFα, IL-1, IL-6, IL-8 and IL-12 following TLRs activation by microbial products supports the role of these receptors in cases of infections accompanied by hyperthermia [16]. It has been reported that TLRs activation is not only temperature- but also time-dependent. Matsui, et al. [17] have found that in TLR2 activated microglia the production of TNFα, and IL-10 was time-dependently enhanced with temperature elevation. The results of our study indicate that the exposure time to hyperthermia, i.e. 4 versus 24 hours at 40°C played a certain role in the extent of cytokine production. However, the difference in cytokine generation was more pronounced with stimulated cells. The discernible decrease of all cytokines examined after 24 hrs of incubation at 40°C is of interest, since the increase cytokine level in patients with inflammatory processes accompanied with high fever may exert undesirable effect [18]. This point emphasizes the intricate mechanism linked to the immune defence and the need to maintain a balance in production of pro- and anti-inflammatory cytokines by immune cells during their confrontation with pathogens.
In short, human unstimulated and stimulated PBMC exposed to 40°C for 4 and 24 hrs showed a time-dependent modulation of inflammatory cytokine expression compared to cells incubated at 37°C for the same time periods.