The CD56+ cells freshly isolated from human peripheral blood contain a substantial subset of CD14+CD86+HLA-DR+ cells which spontaneously differentiate into enlarged DC (dendritic cell)-like cells. We show here that interferon-a (IFN-α)-induced DC-like cells expressing high levels of CD56 and CD14 (here after, CD56high+IFN-DCs) can be differentiated from monocytes obtained as adherent cells from patients with various cancers, in the presence of IFN-α and granulocyte-macrophage colony stimulating factor (GM-CSF) in vitro. These cells expressed high levels of CD14, CD86, and HLA-DR and may be an in vitro counterpart of the circulating CD14+CD86+HLA-DR+ cells in blood. In contrast to conventional mature IL-4DCs which are differentiated from monocytes as adherent cells in the presence of GM-CSF, IL-4, and TNF-α (hereafter, mIL-4DCs), the CD56high+IFN-DCs exhibited a stronger capacity in stimulating autologous CD56+Vγ9γδT cells expressing high levels of CD16 which produced high amounts of IFNγ and TNF-α, in comparison with the mIL-4DCs. Intravenous administration of a large number of Vγ9γδT cells activated by CD56high+IFN-DCs pulsed with zoledronate (here after, CD56high+IFN-DC-activated Vγ9γδT cells) to patients with a non-small cell lung cancer resulted in the increase of PB Vγ9γδT cells among which CD56+Vγ9γδT cells and CD16+Vγ9γδT cells predominantly increased in vivo. Among γδT cell-based immunotherapy, vaccination with CD56high+IFN-DC-activated Vγ9γδT cells may lead to improved clinical outcomes of patients with cancer.
CD56high+IFN-DC, CD56+ Vγ9γδT, Zoledronate, Immunotherapy
DC: Dendritic Cell, IFNa: Interferon-a, GM-CSF: Granulocyte/Macrophage-Colony Stimulating Factor, mIL-4DCs: Mature DCs Generated with IL-4, GM-CSF, and TNF-α, IFN-DCs: DCs generated with IFN-α and GM-CSF, CD56high+IFN-DCs: IFN-DCs expressing high levels of CD56
γδT cells are unconventional T cells playing a major role in innate immune responses against microbes, stressed cells, and tumor cells [1]. In humans, most of our knowledge about the specificity and biological role of γδT cells is derived from analysis of a major peripheral subset referred to as Vγ9Vδ2T cells, comprising 5-10% of all circulating T cells [2]. Unlike classical aß T cells, the Vγ9Vγ2T cells can interact with low molecular mass phosphate-containing, non-processed antigens, such as pyrophosphomonoesters and alkyl amine in an HLA independent manner [3,4]. Aminobisphosphonates, such as pamidronate and zoledronate, which were originally developed as therapeutic drugs for osteoporosis but are increasingly used for cancer therapy, have also been shown to activate Vγ9γδT cells in an HLA independent manner, and the aminobisphosphonate-activated Vγ9γβT cells were functionally characterized in vitro and in vivo [5-7]. In contrast to pyrophosphomonoesters, the Vγ9γδT cell-activation by aminobisphosphonates is highly dependent on the presence of antigen presenting cells (APCs) including monocytes and DCs [8,9]. Aminobisphosphonates appear to target the mevalonate pathway of the APCs and induce the accumulation of phosphorylated metabolities, such as isopentenyl pyrophosphates, which are recognized by the Vγ9γδT cell.
It is well established that human Vγ9γδT cells can recognize ligands expressed by tumor cells and that they exert strong cytotoxic activity against tumor cell lines [10-12]. The activated Vγ9γδT cells rapidly release Th1 cytokine such as IFNa and TNFγ, enhancing antitumor activity by inhibiting tumor growth and activating components of the adaptive immune system [13,14]. Recently, adoptive transfer of ex vivo-expanded autologous Vγ9γδT cells is being considered for development as a new immunotherapeutic approach for patients with cancer. The clinical trials with immunotherapy using Vγ9γδT cells for patients (Pts) with cancers, including multiple myeloma [15], non-small cell lung cancer (NSCLC) [16], renal cell carcinoma [17] and a variety of metastatic solid tumors [18] have been performed. In many cases with clinical trials, a large number of Vγ9γδT cells were expanded from PBMCs using zoledronate with high concentration of IL-2, however, the clinical responses were seen in a minority of patients, presumably, at least in part, as a result of insufficient expansion of Vγ9γδT cells possessing high effector function capable of eradicating tumor cells. Therefore, one of goals of an effective immunotherapy using Vγ9γδT cells against cancers is the induction of Vγ9γδT cells which have high effecter functions against tumor cells.
Among Vγ9γδT cells, CD56+Vγ9γδT cells have higher production of IFNγ and TNFγ and higher cytotoxicity against tumor cells, in comparison with CD56-Vγ9γδT [13]. We have recently reported that among DCs, CD56high+DCs which are differentiated from monocytes obtained as adherent cells derived from healthy donors (HDs) and patients with metastatic melanoma in the presence of IFN-αlpha (IFNβ) and GM-CSF (referred to as CD56high+IFN-DCs) have greater capacity to expand CD56+Vγ9γδT cells which produce high amounts of IFNγ in comparison with the most commonly used mature IL-4DCs which are differentiated from monocytes obtained as adherent cells in the presence of IL-4, GM-CSF and TNFγ and do not express CD56 (referred to as mIL-4DCs) [19]. On the basis of these previous studies, it has been speculated that CD56high+DCs can be differentiated from monocytes of patients with various cancers and they preferentially expand CD56+Vγ9γδT cells which produce high amounts of IFNγ and TNFα in the presence of zoledronate in vitro, and that the infusion of Vγ9γδT cells activated by CD56high+DCs in the presence of zoledronate and IL-2 (referred to as CD56high+DC-activated Vγ9γδT cells) may result in the preferential induction of CD56+Vγ9γδT cells in vivo.
The aims of the present study are as follows: 1) to confirm whether CD56high+IFN-DCs can be differentiated from monocytes obtained as adherent cells from Pts with various cancers, whether the CD56high+IFN-DCs preferentially expand CD56+Vγ9γδT cells which produce high amounts of IFNγ and TNFα in comparison with the mIL-4DCs in the presence of zoledronate and IL-2 and whether intravenous infusions of CD56high+DC-activated Vγ9γδT cells to the Pts effectively induce CD56+Vγ9γδT cells in PB in vivo.
The surface phenotypes were determined by single to five-color flow cytometry using an Epics XL MCL (Beckman Coulter, BC, CA). The following monoclonal antibodies (mAbs) were purchased from BC: anti-CD3(UCHT1), anti-Vγ9TCR(IMMU 360), anti-CD14(RM052), anti-CD16(3G8), anti-CD45(J.33), anti-CD54(84H10), anti-CD56(N901), anti-CD69(TPI.55.3), anti-HLADR(Immu-357), anti-CD80(MAB104), anti-CD86(HA5.2B7), anti-CD40(MAB89), anti-CD11c(BU15), anti-CD83(HB15a), anti-CD36(FA6.152), mouse IgG1(679.1Mc7), mouse IgG2a (7T4-IF5) and mouse IgG2b(A-1) mAbs. Anti-HLA-AB(G46-2.6) and anti-CCR7 (150503) mAbs were purchased from Beckton Dickinson (San Jose, CA) and R & D System (Minneapolis, MN), respectively. All mAbs were conjugated with FITC, PE, ECD, PC5, or PC7. Zoledronate was purchased from Novartis Pharmaceuticals (Basel, Switzerland).
PBMCs were isolated from HDs and Pts with cancer including NSCLC, metastatic melanoma, cervical cancer, metastatic breast cancer, metastatic pancreatic cancer, metastatic sarcoma, and multiple myeloma. The in vitro study was approved by the Ethics Committee of the Biotherapy Institute of Japan and the Research Ethics Committee of the Queensland Institute of Medical Research, the University of Queensland. The in vivo study was approved by the Ethics Committee of the Biotherapy Institute of Japan. All the subjects provided written informed consent to use their PB. For cell culture in this study, AIM-V medium (Invitrogen, Tokyo, Japan) containing 10% heat-inactivated human AB serum was used. PBMCs separated by density gradient centrifugation with Lymphoprep (Nycomed, Oslo, Norway) were suspended in the medium and then incubated in a flask (Corning Incorporated, Japan) for 2 h at 37°C. After removing nonadherent cells, adherent cells were cultured in a fresh medium containing GM-CSF (1000U/mL; Primmune Inc., Japan) and IFN-α (1000U/mL; INTRON, MSD K.K., Japan) for 3 days to obtain CD56high+IFN-DCs. Alternatively, adherent cells were cultured for 5 days in the medium containing GM-CSF (1000U/mL; Primmune Inc., Japan) and IL-4 (500U/mL; Primmune Inc., Japan) to obtain IL-4DCs. The cells were further cultured for 2 days in the presence of TNFγ (10ng/mL: Peprotech Ltd., UK) to obtain mIL-4DCs. In the previous our study [19], we compared monocytes obtained as adherent cells with purified CD14+ cells as starting materials. The results showed that the expression levels of CD56 on adherent cell-derived IFN-DCs were higher than that on purified CD14+ monocyte-derived IFN-DCs and then, we speculated that CD56high+IFN-DCs preferentially expand CD56+Vγ9γδT cells through adhesion molecules, CD56 [20]. Thus, we used adherent cells as starting materials in this study as well.
For induction of Vγ9γδT cells, lymphocytes (2x106) as non-adherent cell fraction were cultured with autologous CD56high+IFN-DCs or mIL-4DCs (2x105) in the presence of zoledronate (5µM) and IL-2 (1000U/mL). The percentage of Vγ9γδT cells among the expanding lymphocytes, and the percentages of CD56+, CD16+, and CD69+cells among the expanding Vγ9γδT cells, were assessed by flow cytometry using a combination of mAbs described above.
The Vγ9γδT cells were firstly expanded from PBMCs from the patients in the presence of zoledronate (10 µM) and IL-2 (1000U/mL). Then, the PB lymphocytes containing high percentages of Vγ9γδT cells were activated by autologous CD56high+IFN-DCs for approximately 24 hours in the presence of zoledronate (5 µM) and IL-2 (1000U/mL) to obtain CD56high+IFN-DC-activated Vγ9γδT cells. Finally, a large number of CD56high+IFN-DC-activated Vγ9γδT cells were infused to the patients. The reason why the Vγ9γδT cell-rich fraction were used as responding cells, instead of PBMCs wherein the percentage of Vγ9γδT cells among PBMCs were less than 10%, was because of aiming to obtain a large number of CD56high+IFN-DC-activated Vγ9γδT cells as many as possible.
Following culture of lymphocytes with autologous CD56high+IFN-DCs from cancer patients in the presence of zoledronate and IL-2 for 10 days, the expanded lymphocytes among which the mean percentages of Vγ9γδT cells from cancers patients including two NSCLC patients and two metastatic melanoma patients were 86.1 ± 9.1%, were separated into Vγ9γδT cells expressing high levels of CD56 (fraction A) (the mean percentage of CD56+ Vγ9γδT cells among Vγ9γδT cells was 98.8% ± 0.2% from three Pts) and Vγ9γδT cells expressing low levels of CD56 (fraction B) (the mean percentage of CD56+ cells among Vγ9γδT cells was 8.0 ± 2.5%) using CD56 mAb-conjugated microbeads (Miltenyi Biotech). Each fraction, A or B was treated as described previously [13]. Then, each fraction was stained for surface markers of Vγ9γδT cells using anti-CD3-ECD, anti-CD56-PC5, and anti-Vγ9TCR-FITC mAbs. For the intracytoplasmic staining of cytokines, the each fraction was also treated as described previously [13]. Finally, each fraction was stained anti-IFNγ-PE or anti-TNFγ-PE mAb (BC) for 20 min at 4oC, washed with PBS containing 1% bovine serum albumin, and analyzed for the productions of IFNγ and TNFγ using flow cytometry.
To evaluate the involvement of CD56 in the preferential expansion of CD56+ Vγ9γδT cells by CD56high+IFN-DCs, lymphocytes were cultured with CD56high+IFN-DCs in the presence of zoledronate and IL-2 with anti-CD56mAb (clone N901; Coulter Clone) or an isotype control, mouse IgG1 (clone 2E12) (MBL) at 10µg/mL each.
P values were calculated using the paired/two-tailed Student's t-test and differences were considered significant at a p value < 0.05 and highly significant at a p value < 0.01.
Characterization of the surface molecules on CD56high+IFN-DCs in comparison with those on mIL-4DCs was performed. Representative phenotypes of CD56high+IFN-DCs and mIL-4DCs from HD1 and Pt1 with NSCLC showed that an almost identical phenotypic pattern with mIL-4DCs, regarding HLA-AB, HLA-DR, CD80, CD86, CD40, CD54, CD83 and CCR7 except CD56 and CD14 (Figure 1a,1b). Among cancer patients including Pt. 2 with NSCLC, Pt. 3 with metastatic melanoma, Pt. 4 with cervical cancer, Pt. 5 with metastatic breast cancer, Pt. 6 with metastatic pancreatic cancer, Pt.7 with metastatic sarcoma and Pt. 8 with multiple myeloma. In all patients described above, the percentages of CD56+ cells and CD14+ cells among CD56high+IFN-DCs were higher than those among mIL-4DCs (Figure 2a). Statistical analysis of data from fourteen cancer Pts, including four melanoma Pts, three lung cancer Pts, three multiple myeloma Pts, a cervical cancer Pt, a breast cancer Pt, a pancreatic cancer Pt, and a sarcoma Pt showed that the percentages of CD56+ cells and CD14+ cells among CD56high+IFN-DCs were significantly higher than those among mIL-4DCs [58.8 ± 14.7 vs 1.8 ± 1.6, p< 0.00001 for CD56+ cells and 89.8 ± 5.3 vs 5.2 ± 9.6, p< 0.00001 for CD14+ cells (Figure 2b)].
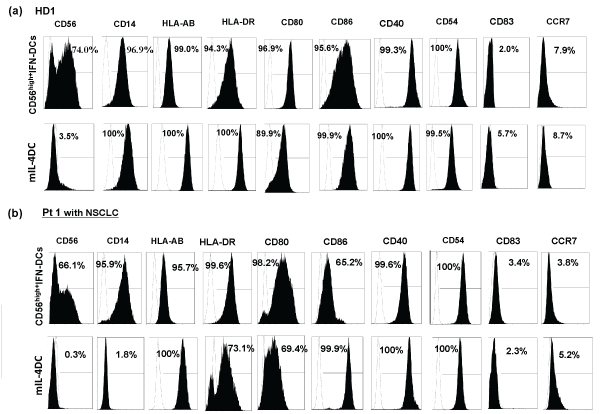 Figure 1: As representative data, phenotypic patterns of CD56high+IFN-DCs and mIL-4DCs derived from HD1 (a) and Pt1 with non-small cell lung cancer
(NSCLC) (b) were shown.
View Figure 1
Figure 1: As representative data, phenotypic patterns of CD56high+IFN-DCs and mIL-4DCs derived from HD1 (a) and Pt1 with non-small cell lung cancer
(NSCLC) (b) were shown.
View Figure 1
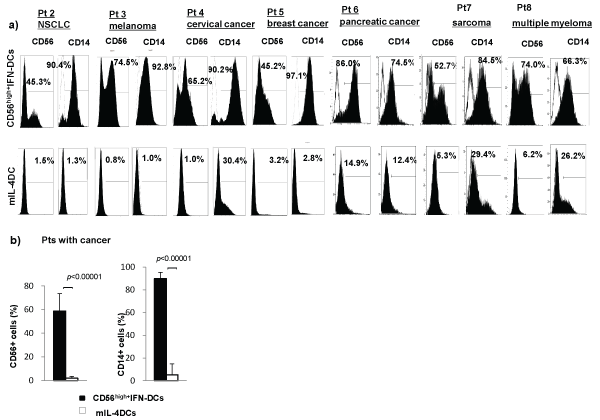 Figure 2: (a) Phenotypic patterns of CD56 and CD14 on CD56high+IFN-DCs and mIL-4DCs derived from various cancer patients, including Pt2 with NSCLC, Pt 3 with melanoma, Pt 4 with cervical cancer, Pt5 with breast cancer, Pt6 with pancreatic cancer, Pt 7 with sarcoma, and Pt 8 with multiple myeloma (b) Statistical analysis of data from fourteen cancer Pts showed that the percentages of CD56+ cells and CD14+cells among CD56high+IFN-DCs were significantly higher than those on mIL-4DCs [for CD56; (p< 0.00001) and for CD14; (p< 0.00001)].
View Figure 2
Figure 2: (a) Phenotypic patterns of CD56 and CD14 on CD56high+IFN-DCs and mIL-4DCs derived from various cancer patients, including Pt2 with NSCLC, Pt 3 with melanoma, Pt 4 with cervical cancer, Pt5 with breast cancer, Pt6 with pancreatic cancer, Pt 7 with sarcoma, and Pt 8 with multiple myeloma (b) Statistical analysis of data from fourteen cancer Pts showed that the percentages of CD56+ cells and CD14+cells among CD56high+IFN-DCs were significantly higher than those on mIL-4DCs [for CD56; (p< 0.00001) and for CD14; (p< 0.00001)].
View Figure 2
We firstly examined whether CD56high+IFN-DCs from Pts with various cancers preferentially expand Vγ9γδT cells expressing high levels of CD56 in the presence of zoledronate and IL-2 in vitro.
Representative data from HD1 and five cancer Pts following culture of lymphocytes with CD56high+IFN-DCs or mIL-4DCs for 8-14 days, were shown in Figure 3a. Before culture, the percentage of Vγ9γδT cells among lymphocytes was 8.6% for HD1, 9.6% for Pt.1, 9.3% for Pt2, 3.5% for Pt3, 4.6% for Pt4, and 3.9% for Pt7.
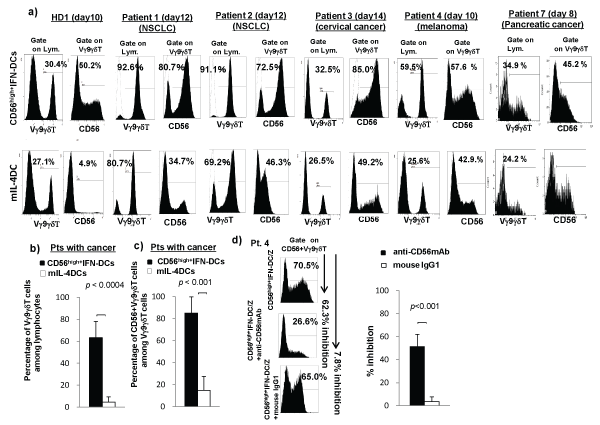 Figure 3: Preferential expansion of CD56+Vγ9γδT cells by CD56high+IFN-DCs in the presence of zoledronate and IL-2. Lymphocytes as non-adherent cells were cultured with autologous CD56high+IFN-DCs and IL-2 for 8 to 14 days in the presence of IL-2 and zoledronate. (a) Representative data of preferential expansion of CD56+Vγ9γδT cells by CD56high+IFN-DCs derived from HD1, Pt1 with NSCLC, Pt2 with NSCLC, Pt3 with cervical cancer, Pt4 with melanoma and Pt7 with pancreatic cancer, in comparison with that by mIL-4DCs, are shown. Statistical analysis of data from fourteen cancer patients showed that the percentage of Vγ9γδT cells among lymphocytes activated by CD56high+IFN-DCs was significantly higher than that expanded by mIL-4DCs (p< 0.0004) (b) and that the percentage of CD56+ Vγ9γδT cells among Vγ9γδT cells expanded by CD56high+IFN-DCs was significantly higher than that expanded by mIL-4DCs (p< 0.001) (c). As a representative result from Pt4 with melanoma, the preferential expansion of CD56+Vγ9γδT cells by CD56high+IFN-DCs in the presence of zoledronate and IL-2 was partially blocked by anti-CD56mAb, but not by isotype control (d; left histograms). Statistical analysis of data from three Pts with melanoma showed that the percent inhibition by anti-CD56mAb was significantly higher than the by mouse IgG1 (d; right columns). Percent inhibition was calculated as follows: the percentage of expanded CD56+Vγ9γδT cells minus the percentage of expanded CD56+Vγ9γδT cells in the presence of anti-CD56 mAb of mouse IgG1 divided by the percentage of expanded CD56+Vγ9γδT cells x 100.
View Figure 3
Figure 3: Preferential expansion of CD56+Vγ9γδT cells by CD56high+IFN-DCs in the presence of zoledronate and IL-2. Lymphocytes as non-adherent cells were cultured with autologous CD56high+IFN-DCs and IL-2 for 8 to 14 days in the presence of IL-2 and zoledronate. (a) Representative data of preferential expansion of CD56+Vγ9γδT cells by CD56high+IFN-DCs derived from HD1, Pt1 with NSCLC, Pt2 with NSCLC, Pt3 with cervical cancer, Pt4 with melanoma and Pt7 with pancreatic cancer, in comparison with that by mIL-4DCs, are shown. Statistical analysis of data from fourteen cancer patients showed that the percentage of Vγ9γδT cells among lymphocytes activated by CD56high+IFN-DCs was significantly higher than that expanded by mIL-4DCs (p< 0.0004) (b) and that the percentage of CD56+ Vγ9γδT cells among Vγ9γδT cells expanded by CD56high+IFN-DCs was significantly higher than that expanded by mIL-4DCs (p< 0.001) (c). As a representative result from Pt4 with melanoma, the preferential expansion of CD56+Vγ9γδT cells by CD56high+IFN-DCs in the presence of zoledronate and IL-2 was partially blocked by anti-CD56mAb, but not by isotype control (d; left histograms). Statistical analysis of data from three Pts with melanoma showed that the percent inhibition by anti-CD56mAb was significantly higher than the by mouse IgG1 (d; right columns). Percent inhibition was calculated as follows: the percentage of expanded CD56+Vγ9γδT cells minus the percentage of expanded CD56+Vγ9γδT cells in the presence of anti-CD56 mAb of mouse IgG1 divided by the percentage of expanded CD56+Vγ9γδT cells x 100.
View Figure 3
Following culture with CD56high+IFN-DCs from Pts with cancer in the presence of zoledronate and IL-2, the percentage of Vγ9γδT cells among lymphocytes activated by CD56high+IFN-DCs was significantly higher than that among lymphocytes activated by mIL-4DCs (Figure 3b) and the percentage of CD56+ Vγ9γδT cells among the Vγ9γδT cells expanded by CD56high+IFN-DCs was also significantly higher than that expanded by mIL-4DCs (Figure 3c). As a representative result from Pt1 with NSCLC, before culture, the total number of CD56+ Vγ9γδT cells among lymphocytes was 1.2 x104 and following culture in the presence of zoledronate and IL-2, the total number of CD56+ Vγ9γδT cells expanded by CD56high+IFN-DCs and mIL-4DCs were 2.9x106 and 1.3x106, respectively, showing that a total number of CD56+γδT cells expanded by CD56high+IFN-DCs was twofold higher than that expanded by mIL-4DCs. These results indicate that CD56high+IFN-DCs were not only required for the development of CD56+ Vγ9γδT effector function, but also for CD56+ Vγ9γδT cell-expansion.
To confirm the involvement of CD56 in preferential expansion of CD56+ Vγ9γδT cells by CD56high+IFN-DCs, the blocking experiment using anti-CD56mAbs was performed. As representative results from Pt4 with melanoma, the percentage of CD56+ Vγ9γδT cells decreased from 70.5% to 26.6% in the presence of anti-CD56 mAb (62.3% inhibition) and to 65.0% in the presence of IgG1 (7.8% inhibition) (Figure 3d, left histograms). Statistical analysis of results from three Pts with melanoma showed that the percent inhibition of CD56+ Vγ9γδT cells in the presence of anti-CD56 mAb was significantly higher than that in the presence of control IgG1 [51.3 ± 10.6% inhibition with anti-CD56 mAb and 3.6 ± 4.1% inhibition with mouse IgG1, p< 0.001 (Figure 3d, right columns)].
Then, we assessed productions of IFNγ and TNFα by CD56+ Vγ9γδT cells in comparison with those by CD56- Vγ9γδT cells. As representative results from Pt. 2 with NSCLC shown in Figure 4a, productions of IFN? and TNFγ by Vγ9γδT cells expressing high levels of CD56 (fraction A; the percentage of CD56+ cells among Vγ9γδT cells was 98.6%) were higher than those by Vγ9γδT cells expressing low levels of CD56 (fraction B; the percentage of CD56+ cells within Vγ9γδT cells was 10.7%). Statistical analysis from four cancer patients including two NSCLC patients and two metastatic melanoma patients showed that IFN? and TNFγ productions by Vγ9γδT cells expressing high levels of CD56 were significantly higher than those by Vγ9γδT cells expressing low levels of CD56 [for IFNγ 57.6 ± 5.5%vs 33.0 ± 2.5, p< 0.03; for TNF-α, 52.9 ± 2.3 vs 31,4 ± 6.4%, p< 0.05 (Figure 4b)].
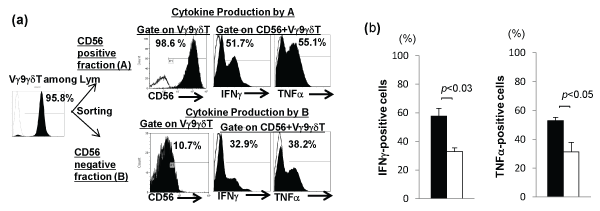 Figure 4: (a) The productions of IFNγ and TNFa by Vγ9γδT cells expressing high levels of CD56 (the percentage of CD56+ cells among Vγ9γδT cells was 98.6%; fraction A) were greater than those by Vγ9γδT cells expressing low levels of CD56 (the percentage of CD56+ cells among Vγ9γδT cells was 10.7%; fraction B) in vitro. (b) Statistical analysis of data from cancer patients including two NSCLC patients and two metastaic melanoma patients showed that the production of IFNγ by CD56+Vγ9γδT cells was significantly higher than that by CD56-Vγ9γδT cells.
View Figure 4
Figure 4: (a) The productions of IFNγ and TNFa by Vγ9γδT cells expressing high levels of CD56 (the percentage of CD56+ cells among Vγ9γδT cells was 98.6%; fraction A) were greater than those by Vγ9γδT cells expressing low levels of CD56 (the percentage of CD56+ cells among Vγ9γδT cells was 10.7%; fraction B) in vitro. (b) Statistical analysis of data from cancer patients including two NSCLC patients and two metastaic melanoma patients showed that the production of IFNγ by CD56+Vγ9γδT cells was significantly higher than that by CD56-Vγ9γδT cells.
View Figure 4
In the case of Pt1 with NSCLC, before culture, the percentage of Vγ9γδT cells was 9.6%, among which the percentages of CD56, CD16, NKG2D, and CD69 were 59.5%, 31.2%, 94.9%, and 0.9%, respectively (Figure 5a). After 14 day-culture of lymphocytes with CD56high+IFN-DCs in the presence of IL-2 and zoledronate, the percentage of Vγ9γδT cells among lymphocytes was 90.2%, among which the percentages of CD56, CD16, NKG2D, and CD69 were 91.2%, 91.7%, 98.6%, and 60.1%, respectively (Figure 5a). These results showed that in the case of Pt1, CD56high+IFN-DC-activated Vγ9γδT cells expressed high levels of CD56, CD16, NKG2D and CD69 in vitro. Note that in our own observations of forty cancer patients, including nine patients with NSCLC, following culture of PBMC in the presence of zoledronate and IL-2 for 14 days, the percentage of Vγ9γδT cells among lymphocytes was 81.8 ± 11.6%, among which the percentages of CD56+ cells and CD16+ cells were 30.1 ± 12.2% and 19.3 ± 12.2%, respectively. These results show that in comparison with using PBMC wherein monocytes play as APCs, the percentages of CD56+ cells and CD16+ cells among CD56high+IFN-DC-activated Vγ9γδT cells wherein DCs act as APCs were higher.
On the basis of these in vitro observations, CD56high+IFN-DC-activated Vγ9γδT cells were infused to Pt. 1 as follows. The patient received 12 intravenous infusions of CD56high+IFN-DC-activated Vγ9γδT cells, in which 2.7 x 109 ± 1.5 x 109 (n=12) of Vγ9γδT cells which were prepared from PBMCs in the presence of zoledronate and IL-2 described in the Materials and Methods, were cultured with 5.8 ± 3.5 x 106 (n=12) of autologous CD56high+IFN-DCs for 24 hours in the presence of zoledronate and IL-2, and then, the obtained CD56high+IFN-DC-activated Vγ9γδT cells were infused to Pt1 at approximately 2 or 3-week intervals. This vaccination is well tolerated by the patient without experiencing any adverse effects or clinical signs of autoimmune reactions. Tumor response was not a focus of this treatment, but it is of interest that following this vaccination sustained decreases in one of serum tumor marker, SLX, were observed. Each assessment was done using PBMCs which were collected just before each infusion. The percentage of Vγ9γδT cells among PB lymphocytes was assessed firstly after two infusions. A total of eleven assessments of Vγ9γδT cells among PB lymphocytes were carried out (Figure 5b). A total of 7 assessments of Vγ9γδT cells among PB lymphocytes, CD56+ Vγ9γδT cells among Vγ9γδT cells, and CD16+ Vγ9γδT cells among Vγ9γδT cells following infusions were carried out (Figure 5c). The percentage of Vγ9γδT cells among PB lymphocytes increased after two infusions from 9.6% (before infusion) to 19% after two infusions and this high percentage maintained following infusions [mean percentage of Vγ9γδT cells among lymphocytes ± SD with 7 infusions was 27.3 ± 4.9%) (Figure 5b,5c, left columns)]. As shown in Figure 5c, middle columns, the percentage of CD56+ Vγ9γδT cells among Vγ9γδT cells increased from 48.4% (before infusion) to 81.2 ± 13.4% after infusions (mean percentage of CD56+ Vγ9γδT cells among Vγ9γδT cells ± SD with 7 infusions). Also, the percentage of CD16+ Vγ9γδT cells among Vγ9γδT cells increased from 25.9% (before infusion) to 69.2 ± 18.1% (mean percentage of CD16+ Vγ9γδT cells among ± SD with 7 infusions), as shown in Figure 5c right columns. Note that the high percentage of Vγ9γδT cells among PB lymphocytes were kept for one month without any cell infusions even though the Pt.1 had chemotherapy drugs such as gefitinib or pemetrexed.
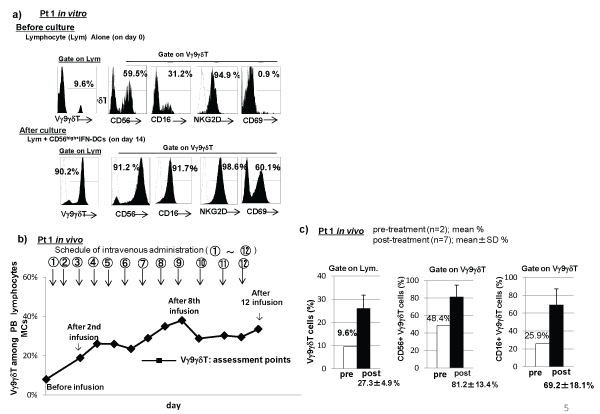 Figure 5: CD56high+IFN-DCs derived from Pt1 with NSCLC effectively expand Vγ9γδT cells in vitro and in vivo. The CD56high+IFN-DCs derived from Pt1 with NSCLC effectively expand Vγ9γδT cells which expressed high levels of CD56, CD16, NKG2D, and CD69, in the presence of zoledronate and IL-2 in vitro (a). The percentages of Vγ9γδT cells among PB lymphocytes and the percentages of CD56+ cells and CD16+ cells among Vγ9γδT cells increased following multiple intravenous infusions of a large number of CD56high+IFN-DCs-activated Vγ9γδT cells to Pt1 (b and c).
View Figure 5
Figure 5: CD56high+IFN-DCs derived from Pt1 with NSCLC effectively expand Vγ9γδT cells in vitro and in vivo. The CD56high+IFN-DCs derived from Pt1 with NSCLC effectively expand Vγ9γδT cells which expressed high levels of CD56, CD16, NKG2D, and CD69, in the presence of zoledronate and IL-2 in vitro (a). The percentages of Vγ9γδT cells among PB lymphocytes and the percentages of CD56+ cells and CD16+ cells among Vγ9γδT cells increased following multiple intravenous infusions of a large number of CD56high+IFN-DCs-activated Vγ9γδT cells to Pt1 (b and c).
View Figure 5
In another case of Pt2 with NSCLC, the percentage of CD56+ cells and CD14+ cells within CD56high+IFN-DCs were 45.3% and 90.4%, respectively (Figure 2a), and the expression levels of other molecules were similar to those on CD56high+IFN-DCs from Pt1 (data not shown). Before culture, the percentage of Vγ9γδT cells among PB lymphocytes was 9.3 % (Figure 6a). Following culture of PB lymphocytes with autologous CD56high+IFN-DCs from Pt2 for 14 days in vitro, the percentage of Vγ9γδT cells within PB lymphocytes was 97.6%, among which the percentages of CD56+, CD16+, NKG2D+ and CD69+ cells were 85.2%, 91.7%, 98.6% and 60.1%, respectively (Figure 6a). These results also show that CD56high+IFN-DCs effectively activate Vγ9γδT cells expressing high levels of CD56, CD16, NKG2D, and CD69 in vitro.
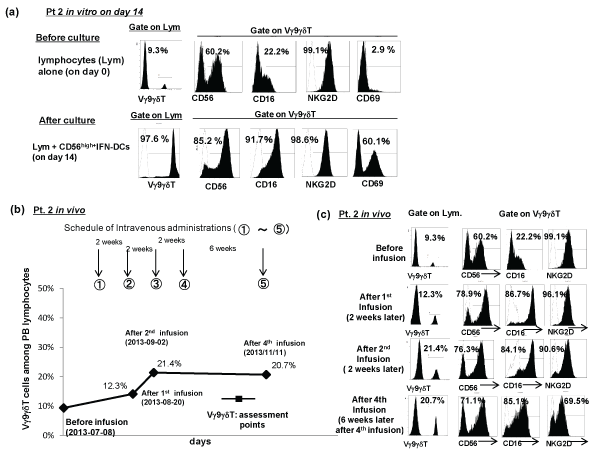 Figure 6: The CD56high+IFN-DCs derived from Pt2 with NSCLC effectively expand Vγ9γδT cells in vitro and in vivo. The CD56high+IFN-DCs derived from Pt. 2 with NSCLC effectively expand Vγ9γδT cells which expressed high levels of CD56, CD16, NKG2D, and CD69, in the presence of zoledronate and IL-2 in vitro (a). The percentages of Vγ9γδT cells among PB lymphocytes and the percentages of CD56+ cells and CD16+ cells among Vγ9γδT cells increased following
multiple intravenous infusions of a large number of CD56high+IFN-DCs-activated Vγ9γδT cells to Pt. 2 (b and c).
View Figure 6
Figure 6: The CD56high+IFN-DCs derived from Pt2 with NSCLC effectively expand Vγ9γδT cells in vitro and in vivo. The CD56high+IFN-DCs derived from Pt. 2 with NSCLC effectively expand Vγ9γδT cells which expressed high levels of CD56, CD16, NKG2D, and CD69, in the presence of zoledronate and IL-2 in vitro (a). The percentages of Vγ9γδT cells among PB lymphocytes and the percentages of CD56+ cells and CD16+ cells among Vγ9γδT cells increased following
multiple intravenous infusions of a large number of CD56high+IFN-DCs-activated Vγ9γδT cells to Pt. 2 (b and c).
View Figure 6
In the case of Pt2, the patient received 5 infusions of CD56high+IFN-DC-activated Vγ9γδT cells wherein 1.7 ± 0.4 x 109 of Vγ9γδT cells (n=5) were activated by 5.8 ± 3.5 x 106 of CD56high+IFN-DCs (n=5) for 24 hours in the presence of IL-2 and zoledronate. The results showed that among five infusions, three immunological assessments (after first, second, and 4th infusion) were done. The results are shown in Figure 6b and Figure 6c. Before infusion, the percentage of Vγ9γδT cells among PB lymphocytes was 9.3% which increased to 12.3% after 1st infusion and 21.4% after 2nd infusion and 20.7% after 4th infusion (Figure 6b). As shown in Figure 6c, before infusion, the percentages of CD56+, CD16+, and NKG2D+ cells among Vγ9γδT cells were 60.2%, 22.2%, and 99.1%, respectively (Figure 6c). On 2 weeks later after 1st infusion, the percentages of CD56+, CD16+, and NKG2D+ cells among Vγ9γδT cells were 78.9%, 86.7%, and 96.1% and these high percentages were kept following 2nd infusion and 4th infusion (Figure 6c). Note that even though there were no infusions for approximately 9 weeks (after 2nd infusion and before 5th infusion), the percentages of CD56+, CD16+, and NKG2D+ cells among Vγ9γδT cells were kept high (Figure 5b)
In this study, we found that CD56high+IFN-DCs which may be a counterpart of circulating CD56+DC-like cells in human blood [21], were differentiated from monocytes as adherent cells from Pts with various cancer in the presence of IFN-α and GM-CSF in vitro (Figure 1). The CD56high+IFN-DCs derived from the Pts preferentially expanded CD56+ Vγ9γδT cells in the presence of zoledronate and IL-2 (Figure 3c). In particular, in the case of Pt1 and Pt2 with NSCLC, the CD56high+IFN-DCs preferentially expand Vγ9γδT cells which expressed high levels of CD56, CD16, NKG2D, and CD69 in vitro (Figure 5a and 6a). Furthermore, the productions of IFN? and TNFγ by Vγ9γδT cells expressing high levels of CD56 were greater than those by Vγ9γδT cells expressing low levels of CD56 in vitro (Figure 4). Taken together, these results with the previous study [19,21] indicate that CD56high+IFN-DCs which are phenotypically and functionally analogous to circulating CD56+DC-like cells in human blood from healthy donors can be differentiated from various cancer Pts and these CD56high+IFN-DCs preferentially expand Th1-type CD56+Vγ9γδT cells which have higher effector functions in comparison with CD56-Vγ9γδT cells. Furthermore, intravenous infusions of CD56high+IFN-DC-activated Vγ9γδT cells to the Pts with NSCLC induce the increase of Vγ9γδT cells expressing high levels of CD56 in PB lymphocytes which may associate with high productions of Th1-type cytokines in vivo.
It is well known that, antigen receptors and adherent molecules are rearranged into a distinct concentration ring known as the immune synapse which is important for promoting activation signaling [22]. CD54 or ICAM-1 is a ligand for the leukocyte integrin complex CD11a/CD18 (LFA-1) and is expressed at different levels on a variety of cells, including APCs, T cells, and B cells [23-25]. The adhesive interaction between CD54 and LFA-1 facilitates cell-cell contact [25] and is thought to strengthen the immune synapse [26]. In the previous study [19], we discussed that the expression levels of CD54 on CD56high+IFN-DCs and mIL-4DCs were the same and therefore, we speculated that another homophilic adhesion molecule, CD56, may contribute in preferential expansion of CD56+Vγ9γδT cells by CD56high+IFNDCs and showed that the expansion of CD56+Vγ9γδT cells by CD56high+IFN-DCs derived from HDs was partially blocked by anti-CD56mAb. In this study, we showed that the expansion of CD56+Vγ9γδT cells by CD56high+IFN-DCs derived from Pts with melanoma was also partially blocked with anti-CD56mAb (Figure 3d) and re-confirmed that CD56high+IFN-DCs may form a strong immune synapse with CD56+Vγ9γδT cells through CD56, resulting in the preferential proliferation of CD56+ Vγ9γδT cells.
Furthermore, it is an interesting observation that the expanded Vγ9γδT cells express high levels of CD16 in addition to CD56, showing NK phenotype. Therefore, the Vγ9γδT cells activated by CD56high+IFN-DCs pulsed with zoledronate may have antibody-dependent-cytotoxic-effects for consideration of immunotherapy combined with monoclonal antibody drugs; for example, combination with cetuzimab for Pts with NSCLC.
It has been shown that like DCs, Vγ9γδT cells present antigens to aß T cells and play APCs [27,28]. In this study we confirmed that CD56high+IFN-DC-activated Vγ9γδT cells expressed antigen presenting molecules, such as HLA-class I, HLA-DR, CD86, and CD40 on the Vγ9γδT cells. in vivo study for Pt1 and Pt2 showed that following infusions of CD56high+IFN-DC-activated Vγ9γδT cells, the percentage of CD56+Vγ9γδT cells within PB lymphocytes increased after two infusions and maintained high percentage of CD56+Vγ9γδT cells during treatments. The reason to maintain high percentage of CD56+Vγ9γδT cells among PB lymphocytes in vivo may be that during culture of Vγ9γδT cells from PBMCs in the presence of zoledronate and IL-2, the expanding Vγ9γδT cells were continuously affected by zoledronate through the culture period and therefore, CD56high+IFN-DC-activated Vγ9γδT cells may act as APCs which express high levels of isopentenyl pyrophosphate on their surface and directly stimulate PB Vγ9γδT cells in vivo without further addition of zoledronate.
These in vivo observations for patients with NSCLC give us an important implication with immunotherapy for cancer Pts. Both Pts 1 and 2 had chemotherapy during immunotherapy with CD56high+IFN-DC-activated Vγ9γδT cells, however, high percentages of CD56+ Vγ9γδT cell and CD16+ Vγ9γδT cells among Vγ9γδT cell were maintained 4 to 8 weeks without the immunotherapy. In general, the percentage of Vγ9γδT cells among PB lymphocytes is lower in patients with cancer and varies between cancer Pts (2.2 ± 2.1%, n=45; in our unpublished observations). In contrast, the percentage of Vγ9γδT cells among PB lymphocytes from patients with NSCLC is similar to that from HDs (5 to 10%). Therefore, the expansion of Vγ9γδT cells from PBMCs of Pts with NSCLC is easier than that from PBMCs of other cancer patients and then, finally a large number of CD56high+IFN-DC-activated Vγ9γδT cells were obtained from Pts with NSCLC.
In this study we have conducted immunotherapy using CD56high+IFN-DC-activated Vγ9γδT cells for only two patients with NSCLC. Although we surely need further extensive in vivo study using CD56high+IFN-DC-activated Vγ9γδT cells for cancer Pts, it can be emphasized that the immunotherapy using CD56high+IFN-DC-activated Vγ9γδT cells which induce CD56+Vγ9γδT cells in vivo may be very useful in cancer patients, in particular in patients who cannot find optimal tumor antigen (s).
Further advantages to use CD56high+IFN-DCs include a feasible and reliable protocol based on clinical grade reagents such as GM-CSF and IFNa for preparation of DCs with shorter culture period, 3 days. These advantages with CD56high+IFN-DCs may have practical implications for the therapeutic use of CD56high+IFN-DCs. In our study, we used adherent cells as monocyte fractions because in comparison with using purified CD14+ monocytes, the expression levels of CD56 on CD56high+IFN-DCs derived from adherent cells are higher than that on CD56high+IFN-DCs derived from CD14+ purified monocytes, as shown in the previous study [19].
In summary, CD56high+IFN-DCs which may be the in vitro counterpart of circulating CD56+DC like cells were differentiated from monocytes as adherent cells of Pts with various cancers, and these CD56high+IFN-DCs preferentially expand Vγ9γδT cells expressing high levels of CD56, CD16, and CD69 in the presence of zoledronate and IL-2. The Vγ9γδT cells expressing high levels of CD56 showed higher effector function such as greater productions of IFN? and TNFγ than those by Vγ9γδT cells expressing low levels of CD56 in vitro. Multiple intravenous infusions of CD56high+IFN-DC-activated Vγ9γδT cells to the Pts with NSCLC resulted in the increase of CD56+Vγ9γδT cells expressing high levels of CD56 and CD16 in PB lymphocytes in vivo which may associate with a better clinical outcome. Although more intensive in vivo study is needed, immunotherapy using CD56high+IFN-DC-activated Vγ9γδT cells may be a promising modality to treat patients with various cancers.
Mie Nieda: conceptualised, designed and performed experiments; responsible for the acquisition, analysis and interpretation of data; responsible for drafting and revising the manuscript; Hiroshi Terunuma: conceptualised and designed experiments. Yuuta Eiraku: performed experiments and provided key technology; Xuewen Deng: conceptualised and designed experiments; Andrew J. Nicol: conceptualised and designed experimetns; supervised analysis and interpretation of data. We thank Mr. Tsubasa Takane for excellent technical assistance.