Background: Pyoderma gangrenosum (PG) is an uncommon, non-infectious, inflammatory skin disorder affecting individuals of all ages. Research characterizing socioeconomic status (SES) of this patient population is limited. Understanding the SES of a patient population is vital to providing a personalized approach for disease management that addresses potential barriers including transportation, work flexibility, cost of medications, and wound care.
Objective: Determine if socioeconomic status and healthcare insurance coverage differs between patients with PG and other dermatology or healthcare system patient populations.
Methods: A single-institution retrospective study of 255 adults with a history of PG, diagnosed between January 1, 2010 and January 1, 2020 was conducted. PG patients were compared to the following age and sex-matched cohorts: 1) Any dermatology patients without PG, 2) Psoriasis patients, 3) Atopic dermatitis patients, 4) Skin cancer patients, and 5) Any healthcare system patients. Demographic and insurance data were collected. SES was determined based on a previously validated algorithm utilizing primary residence zip code.
Results: Approximately 33% of patients with PG had very low SES, defined by SES index scores in the lowest two gross income deciles, compared to 20% among the general healthcare system patient population. Medicare was the most common primary insurance at 47% of PG cohort. Medicaid was the primary insurance in 14% of PG patients. Uninsured patients comprised 11% of the PG cohort compared to 7.6% of dermatology patients and 4% of the general healthcare system patient population.
Limitations: As a single-site study, results may vary based on practice type and geographic location.
Conclusions: Patients with PG have lower SES and are more likely to have government insurance or no insurance than other medical and dermatology patient populations. Physicians should recognize additional barriers these patients may face in the treatment of a condition that can be difficult to manage.
Pyoderma gangrenosum, Socioeconomic status, Insurance, Comorbidities, Wound healing, Autoimmune, Inflammation, Ulcers, Access to care, Health disparities
Pyoderma gangrenosum (PG) is an uncommon, inflammatory skin disorder characterized by painful, ulcerated nodules or pustules with purulent and hemorrhagic exudate [1,2]. The etiology of the disease remains uncertain, though is theorized to be an exaggerated immune response involving neutrophil dysfunction [1,3,4]. Approximately half of PG cases are associated with an underlying systemic disease, most often inflammatory bowel disease (IBD) and diseases of the joints and blood [1]. Considered a diagnosis of exclusion, PG can be challenging to diagnose early in presentation, which may lead to initial wound care mismanagement, potentially worsening the outcome, delaying recovery, and increasing healthcare costs [5,6]. Management is also complicated by limited research on treatment optimization; currently, treatment includes wound care, pain management, topical and systemic immunosuppression, and compression therapy [5]. Therapies are frequently used in combination which adds to the burden of cost for patients [5]. PG is considered a chronic disease, as > 50% of patients on topical or systemic therapies will still require ongoing therapy. Even with use of more advanced treatments like biologics, complete resolution may take years [5,7,8].
Socioeconomic status (SES) is an important marker influencing a patient's access to care and health information [9] and low SES has been associated with various diseases [4,10-14]. Understanding issues related to managing chronic disease in low SES patients allows care teams to employ targeted strategies that better address individual patient needs [15]. Within dermatology, individuals with psoriasis experience lower rates of employment and significantly greater healthcare costs [16]. Atopic dermatitis patients with lower SES experience worse disease outcomes, suggested to be related to transportation costs, inability to take time off work, lack of affordable childcare, and medication costs [17]. Hidradenitis suppurativa (HS) frequently presents in lower SES populations and patients cite issues with transportation, child care, insurance coverage, work flexibility, obesity, food cost, social support, clothing and comfort costs, medication costs, and wound care costs as factors influencing management and outcomes [15].
The relationship of SES and PG is not well characterized. To our knowledge only one study has examined the association of PG and SES, finding that PG manifesting concurrently with IBD presented more commonly in African Americans (p < 0.05), in patients under 50 years of age (p < 0.05), and in patients of the highest income quartiles (p < 0.05) [4]. However, a greater proportion of patients diagnosed with PG presenting without IBD were among the lower income quartiles (p < 0.05). Their study used income as the single parameter to define SES [4]. The objective of this study is to describe the SES of patients with PG compared to other dermatologic diseases and the general medical patient population and to investigate whether particular disease characteristics are associated with differences in SES.
A retrospective case-control study was performed comparing the SES between PG patients and patients with other dermatologic or non-dermatologic conditions at Wake Forest Baptist Health (WFBH) between January 1st, 2010 and January 1st, 2020. The study was approved by the WFBH Institutional Review Board (IRB, 00064501).
Patients were selected using the i2b2 platform query tool offered by the Clinical and Translation Science Institute (CTSI) at Wake Forest School of Medicine (Winston-Salem, NC). The experimental group consisted of patients with PG designated by ICD-9 and ICD-10 codes, including patients who had other dermatoses in addition to PG. Individuals age ≥ 18 years were included in the study. This group of patients was compared to five other WFBH cohorts of randomly selected patients: 1) Patients who had been seen in the dermatology department for any condition except PG 2) Patients with a diagnosis of psoriasis 3) Patients with a diagnosis of atopic dermatitis 4) Patients with a diagnosis of skin cancer, and 5) All patients in WFBH system. Diagnoses for the psoriasis, atopic dermatitis, and skin cancer comparison cohorts were defined using ICD-9 and ICD-10 codes. Patients with PG were then age-matched and sex-matched to patients in each of the five comparison groups at a ratio of 1:4 (1 PG patient to 4 comparison group patients).
SES was determined for each patient in the study using a previously validated method by Statistics Netherlands and used in other dermatologic published studies examining SES [12,18]. The method for determining a patient's SES involved utilizing 2018 US Census Data stratified by zip code for the following parameters: median household income after tax, median property value, median monthly rent, percent of population living 200% below poverty, percent unemployed and over the age of 16, and an education index [19]. The census parameters from all six groups (experimental group + five comparison groups) were then combined using principal component analysis (PCA). From the PCA output, the first principal component (PC) score for each patient, explaining 46.2% of variance in the census data, was selected as the SES index value. To compare the SES index values between groups, comparison group 5 (patients with any medical diagnosis) was used as the reference group. The patients from comparison group 5 were ranked from smallest PC to largest PC and categorized into deciles. Thus, the range of PC values from the first 102 lowest ranked patients corresponded to the lowest 10% SES index values. Likewise, the PC score range for the next 102 patients corresponded to the second decile and so forth. Then, for the remaining four comparison groups and the experimental group, the number of patients that fell within each PC decile range as determined from comparison group 5 were tabulated. Consequently, if patients with PG were to have a similar SES distribution to the general WFBH medical patient population we would expect approximately 10% of patients with PG to fall within each decile range, but if it differed the distribution would be unequal. Finally, the SES index was summated further into quintiles: 1-2 deciles corresponding to very low SES, 3-4 deciles to low SES, 5-6 deciles to moderate SES, 7-8 deciles to high SES, and 9-10 deciles to very high SES [12].
Individual patient data were collected from electronic medical records at the most recent visit for their respective diagnosis which included age, sex, race, body mass index (BMI), zip code, and insurance type. Additionally, medical records of each patient with PG were reviewed to determine the number and location of lesions, tobacco use, and associated comorbidities at their most recent visit.
Results were analyzed using SAS/STAT® software (version 9.4, SAS Institute, Inc.). Descriptive statistics were obtained for the experimental and comparison groups. Within the PG experimental group, correlations between age and SES index and BMI and SES index were evaluated. Inter-group comparisons of SES index and insurance type (Medicaid, Medicare, private insurance, uninsured) were performed using chi-square tests with alpha = 0.05 for significance. The data extracted for the general WFBH medical patient population was used as the expected values/proportions for the chi-square testing for all inter-group comparisons. The insurance type was not listed in every patients' medical record so insurance type calculations were based on samples of sizes of 254 for patients with PG, 1020 for patients with any dermatology diagnosis, 1017 for patients with psoriasis, 1018 for patients with atopic dermatitis, 1020 for patients with skin cancer, and 1020 for patients in the general WFBH medical population.
A total of 255 patients with PG and 1020 patients in each of the five comparison groups (total: 5100 comparison patients) were included in analysis (Table 1). Patients with PG had a mean (SD) age of 60.3 years (15.3). One hundred eighty-eight patients (73.7%) identified as Caucasian, 183 (71.8%) were female sex and 53 of 235 (22.6%; 20 patients had unreported smoking status) used tobacco products. Patients with PG had a mean (SD) BMI of 32.3 kg/m2 (9.5) that was significantly greater than the comparison groups (p < 0.001; Table 2).
Table 1: Patient groups and sample sizes. View Table 1
Table 2: Patient characteristics among various disease conditions. View Table 2
Most patients with PG had at least one associated comorbidity (175; 68.6%). These were the following reported disease associations: musculoskeletal (26.7%), GI (38.8%), hematologic (6.3%), collagen-vascular (2.7%), cancer (9.8%), and other (27.8%; 20.8% with diabetes mellitus).
Patients with PG were most commonly insured by Medicare (47%) while 28% used private insurance. Medicaid was used by a greater percentage of individuals with PG (14%) than the comparison groups (p < 0.001, Figure 1). More individuals with PG were uninsured (11%) than the comparison groups (p < 0.001, Figure 1).
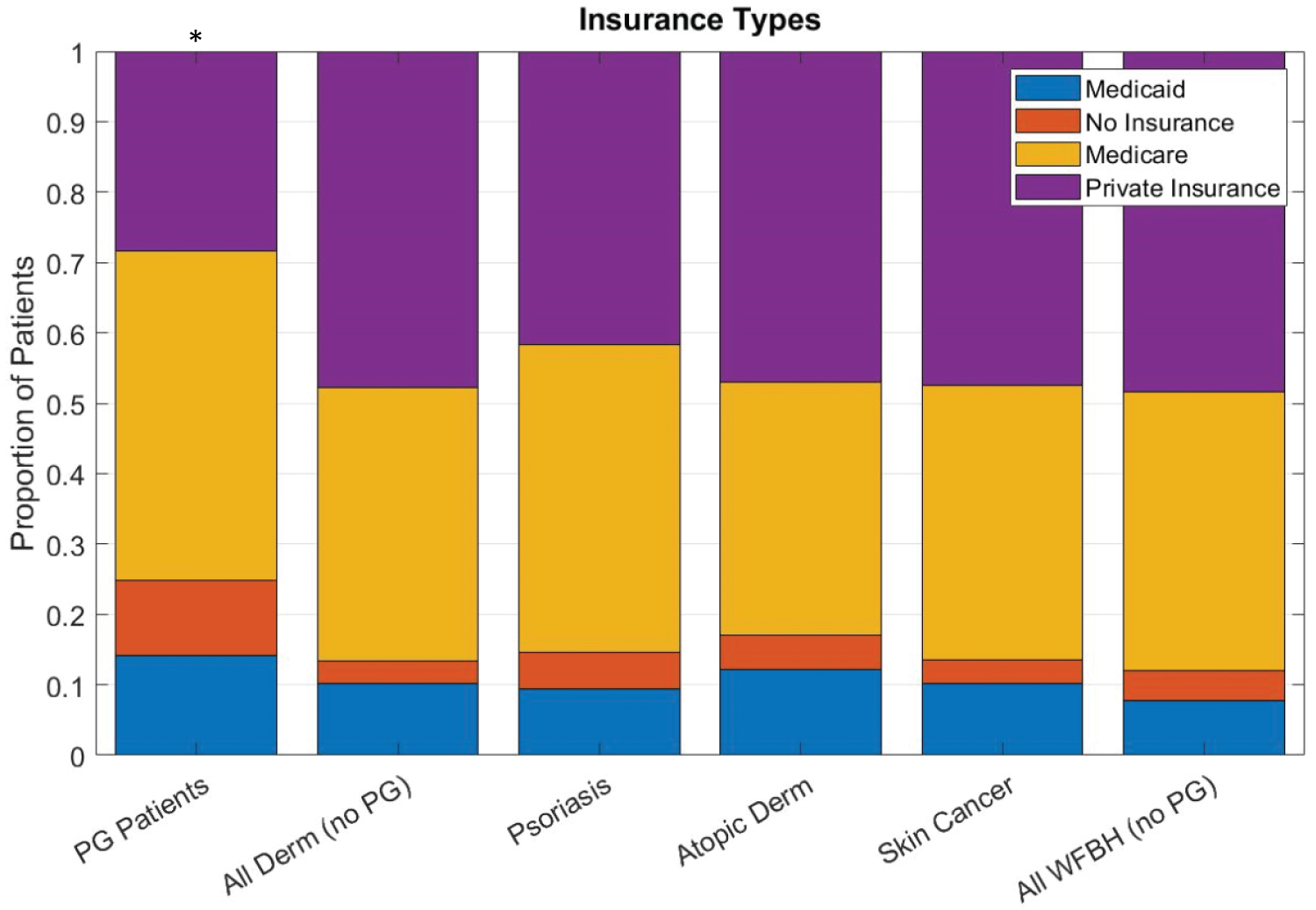 Figure 1: The proportion of PG patients using Medicaid or having no insurance is greater than other patient populations. Asterisk (*) denotes p < 0.001.
View Figure 1
Figure 1: The proportion of PG patients using Medicaid or having no insurance is greater than other patient populations. Asterisk (*) denotes p < 0.001.
View Figure 1
In the PG cohort, 32.6% of individuals had very low SES, more than any of the other cohorts (p < 0.001, Figure 2). The PG cohort also had the least number of patients in the high or very high SES categories compared to the dermatology-related cohorts (p < 0.001, Figure 2).
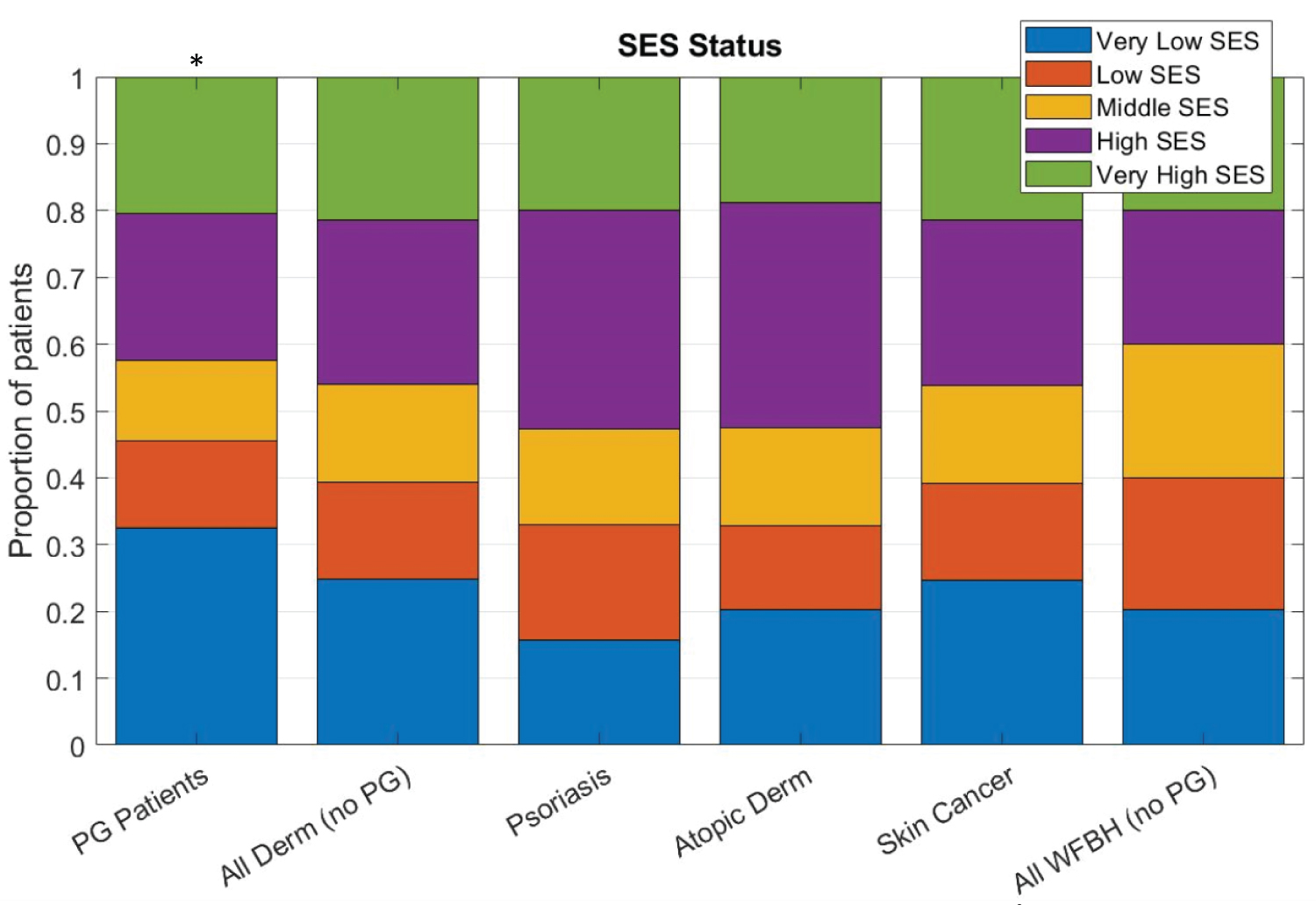 Figure 2: Patients with PG have significantly lower SES than other patient populations. Asterisk (*) denotes p < 0.001. View Figure 2
Figure 2: Patients with PG have significantly lower SES than other patient populations. Asterisk (*) denotes p < 0.001. View Figure 2
Multiple univariate analyses revealed no significant association between BMI, age, lesion number or lesion size and SES index. The distribution of race was not statistically significant between patients with PG and the general WFBH population cohort, though a greater proportion of black individuals and a smaller proportion of white individuals were represented among patients with PG in comparison to the dermatology patient cohort and WFBH population cohort (Table 2).
Individuals with PG were more likely to have Medicaid insurance or be uninsured and have a very low SES, defined by education, unemployment, property value, household income, rent, and poverty level compared to the general medical patient population in this study. There is one previously published study examining SES in hospitalized patients with concurrent IBD and PG. This study found concurrent IBD and PG was associated with the upper third and fourth SES income quartiles (p < 0.05) while those without concurrent IBD were in lower SES quartiles [4]. These researchers excluded the outpatient population and defined SES by income alone while we incorporated both inpatient and outpatient groups and used six parameters to define SES, making for a more robust analysis [20]. Our study highlights a gap in the literature on understanding how SES impacts a patient's risk of developing PG.
Low SES generates persistent activation of a patient's biological stress response system, augmenting their risk for health issues including obesity, hypertension, diabetes, cognitive impairment, and immune dysregulation [21]. Nearly 70% of patients with PG in this study also had an associated comorbidity. PG is associated with conditions including IBD, arthritis, hematologic illnesses, and cutaneous syndromes including the PG, acne, suppurativa hidradenitis (PASH) syndrome [1,22]. Additionally, higher BMI is associated with lower SES, [23] a trend observed in this study and a reported factor contributing to the association between HS and low SES [12]. Moreover, 20% of our patients also have diabetes and research proposed that heightened stress among low SES individuals due to restricted autonomy and altered fat deposition from increased stress hormone levels contribute to increasing the risk of developing type II diabetes mellitus [24]. Therefore, individuals with lower SES may have many of the chronic diseases, inflammatory milieu, and epigenetic variables that could result in the exaggerated inflammatory response seen in PG.
Living with PG is associated with reduced mobility, increased social withdrawal, and increased risk of death three times that of the baseline population rate [25]. The main goals for proper PG management and wound healing include reducing inflammation, limiting pain, and preventing secondary infection [25] which may require coordination between dermatology, wound care, pain management, primary care, and subspecialists for associated-comorbidities. Given that ulcer healing is a slow process over months and occasionally years, [5,8] there is the need for frequent follow-up visits, particularly to monitor for superinfection and medication adherence [25]. Patients with dermatoses often have poor motivation for medication adherence, [26,27] and the added challenges facing individuals with low SES including financial and transportation barriers [28] may exacerbate this behavior. Proper disease management requires significant patient advocacy, the ability to take time off from work, and the financial means to cover copays, medications, and supplies. The inability to work can further compromise income, resources, and insurance status, intensifying barriers to care. The question remains whether patients who have low SES are at greater risk for developing PG lesions due to the heightened inflammatory dysfunction and comorbidities resulting from persistent activation of the stress pathway or whether acquiring PG and its extensive burden of care results in low SES.
Findings of this study may be difficult to extrapolate to other geographic populations as patients were from a single institution and few characterizations of the association between PG and SES have been published. Correlation between SES and disease severity or length of PG course cannot be determined from our data. Further, SES was determined by zip code and insurance type rather than from the patient directly via survey so the SES reported in this study is an approximation. We calculated SES using data from the patient's most recent visit, which was not always the visit at time of diagnosis. This provided a consistent time point for data extraction between experimental and comparison groups, but it is possible that the SES and insurance type of the patient may have differed at initial diagnosis. Additionally, as an academic center, there is a possibility that referral of patients who are uninsured or underinsured from outside practices may introduce a bias potentially skewing results. Age and sex-matching to controls at a 1:4 ratio attempted to address some of these potential confounders.
A greater portion of patients with PG have lower SES than the general patient population. SES is a proxy for access to care and individuals with a lower SES are less likely to receive proper treatment or early diagnoses [29,30]. Consideration of SES when determining clinical management for patients with PG may be prudent. Further research investigating wound healing, length of remission, avoidance of surgery or amputation to determine the impact of SES on PG outcomes would add to our existing knowledge. Replication of these results in other geographic areas would also add support to the correlation of low SES and risk of PG.
Strowd has received grants or support from Galderma, Pfizer, Actelion, and Sanofi-Regeneron. Feldman received research, speaking and/or consulting support from Arcutis, Dermavant, Galderma, GSK/Stiefel, Almirall, Alvotech, Leo Pharma, BMS, Boehringer Ingelheim, Mylan, Celgene, Pfizer, Ortho Dermatology, Abbvie, Samsung, Janssen, Lilly, Menlo, Helsinn, Arena, Forte, Merck, Novartis, Regeneron, Sanofi, Novan, Qurient, National Biological Corporation, Caremark, Advance Medical, Sun Pharma, Suncare Research, Informa, UpToDate and National Psoriasis Foundation. He is founder and majority owner of www.DrScore.com and a founder and part owner of Causa Research, a company dedicated to enhancing patients' adherence to treatment. Huang has received a grant from the Dermatology Foundation. He has received consulting support from Genentech.
Vromans, Williams, and Bashyam have no conflicts to disclose.
IRB: The study was approved by the WFBH Institutional Review Board (IRB, 00064501).
None.