Lymphoma, Non-Hodgkin, Lymphoma, T-cell, cutaneous, Mycosis fungoides, Skin diseases, Vesiculobullous
Mycosis fungoides (MF) is the most common cutaneous lymphoma, accounting for about half of the cases (14), with many clinical and morphological variants (01). One of them is represented by the bullosa form (MFB), which is uncommon, with only 20 cases reported (05) in the literature. We present the case of a young patient whose diagnosis was made through skin biopsy associated with immunohistochemistry (IHC) and immunofluorescence (IF), aiming to discuss the pathophysiological possibilities for this variant [1-4].
A healthy 26-year-old white man, cleaner, presented hypochromic macules of varying sizes distributed throughout the body, sparing his face. In the inguinal and left axillar regions, ex-ulceration-coated erythematous plaques were observed. The initial clinical diagnostic hypotheses were herpetiform dermatitis, IgA dermatitis, and hypochromic MF. Punch biopsies were performed for histopathological examination with immunohistochemistry (IHC) and immunofluorescence (IF).
Histological sections revealed dense dermal lymphocyte infiltrate with cerebriform nuclei, epidermis involvement and Pautrier microabscesses. IHC indicated the immunophenotype T of these lymphocytes, as well as the predominant positivity for CD4 in relation to CD8. The IF was negative for total IgG, IgG, IgM, IgA, C1q and C3, ruling out the possibilities of autoimmune diseases. The diagnosis, after clinical-pathological discussion, was MF. The patient was prescribed topical betamethasone and gentamicin. However, he did not attend follow-up appointments for 11 months. Upon return, the patient reported oscillation in the lesions, and at that time, reported worsening of lesions with vesicles and ulcerations. Clinically, the most severe lesions were in the posterior region of the right forearm and in the left inner thigh, where a clot had formed over an area of the atrophic plaque (Figure 1A, Figure 1B, Figure 1C and Figure 1D).
In this second visit, the new clinical hypotheses were MFB evolved from hypochromic MF, IgA dermatitis, Hailey-Hailey disease, and fixed pigmentary erythema. New biopsies of the two lesions were performed. Laboratory tests and serology came back negative for abnormalities.
The new histological sections showed subepidermal bullae, with atypical lymphocytosis in papillary dermis and epidermis and intra-epidermal microabscesses (Figure 1G). The immunohistochemical study confirmed the T lymphocyte immunophenotyping by CD3 positivity (Figure 1E and Figure 1F). In addition, there was CD4 immunoexpression (Figure 1H) to the detriment of CD8 expression (Figure 1I), with loss of CD7 immunoexpression. The IF was negative for total IgG, IgG, IgM, IgA, C1q and C3. With all the findings, allied to the clinical history, the diagnosis was MFB.
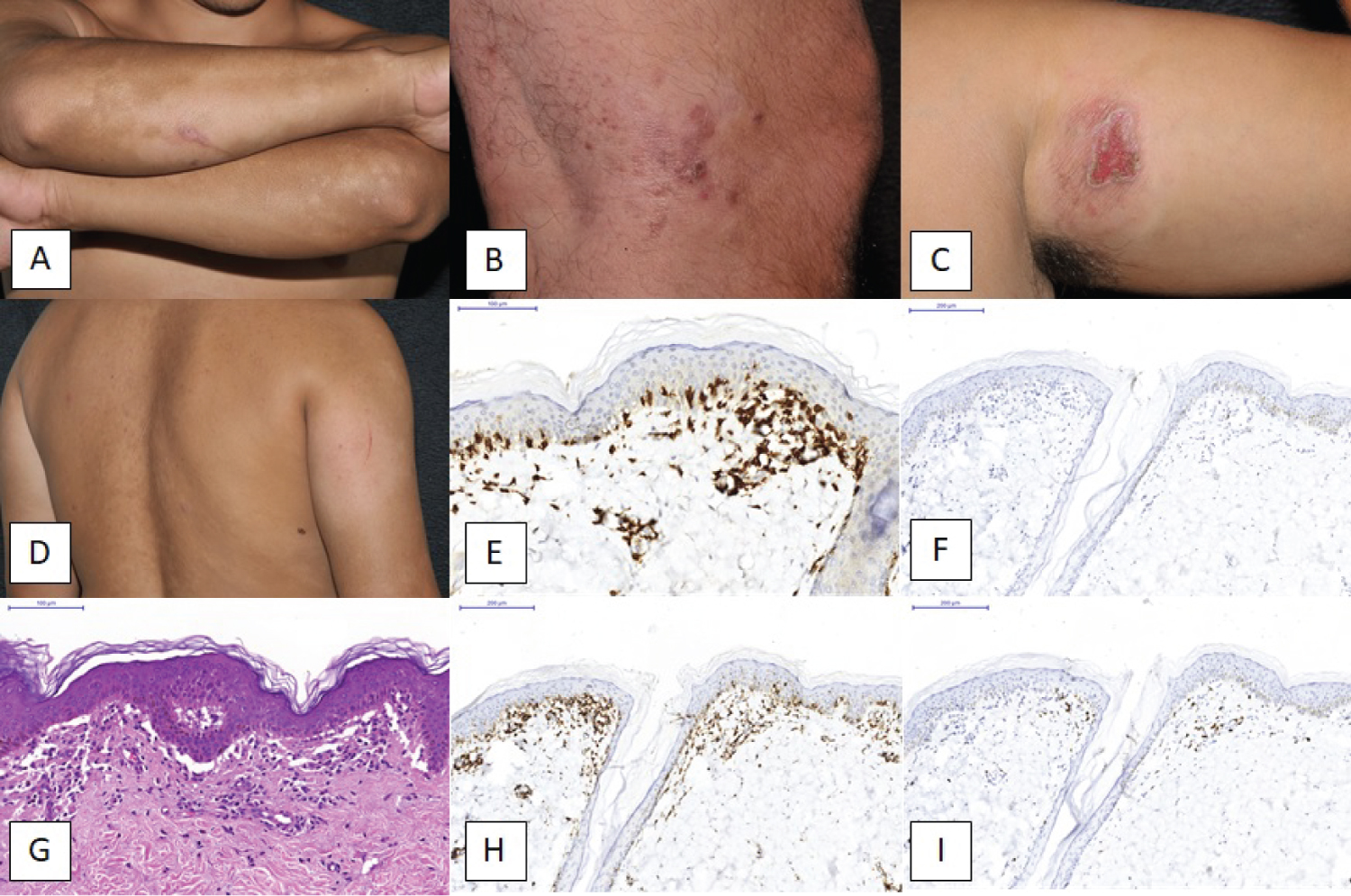 Figure 1: Clinical images (a) Hypochromic macules in his forearms; (b) Small vesicles and macules; (c) An irregular plaque with central lesion. There are some vesicles in periphery of the central ulcerated area; (d) The dorsal area with macules and vesicles; (e) IHC for CD3 (200x); (f) IHC for CD20 (200x); (g) A H&E stain with atypical lymphocytes and a cluster lesion; (h and i) IHC for CD4 (200x) and CD8 (200x), respectively.
View Figure 1
Figure 1: Clinical images (a) Hypochromic macules in his forearms; (b) Small vesicles and macules; (c) An irregular plaque with central lesion. There are some vesicles in periphery of the central ulcerated area; (d) The dorsal area with macules and vesicles; (e) IHC for CD3 (200x); (f) IHC for CD20 (200x); (g) A H&E stain with atypical lymphocytes and a cluster lesion; (h and i) IHC for CD4 (200x) and CD8 (200x), respectively.
View Figure 1
The treatments were photochemotherapy therapy and ultraviolet A radiation (PUVA). After the treatment, the patient presented atrophic plaques replacing blisters, as well as hypochromic macules on the upper limbs and ulcerated erythematous nodule on the posterior aspect of the right thigh. The patient again postponed follow-up for a further 12 months, and, upon return, he was unwilling to adhere to PUVA therapy. However, there was clinical stabilization of the condition even with occasional use of topical medication (Betamethasone plus Gentamycin).
Clinically, MFB makes a differential diagnosis with skin bullosa dermatoses, making the anatomical evaluation decisive. Therefore, if there is such suspicion, it is mandatory first to consider other differential diagnoses, which may occur concomitantly with MF [3-9].
MFB was first described by Garb and Wise in 1943, and the mechanisms of blister formation, which may be intra-epidermal or sub-epidermal, are not yet fully understood. Three proposed mechanisms seem to contribute to these formations: (a) Pautrier micro-abscess confluences, (b) Loss of keratinocyte adhesion with basal lamina due to a proliferation of neoplastic lymphocytes and (c) Lymphocyte production by these same cells would also contribute to the loss of cellular adhesion of keratinocytes (05). The blisters themselves appear months after MF is suspected, with macules, plaques and/or nodules and form on these or healthy skin [7]. The case reported here is interesting because it occurred in a young patient, who is not within the typical age group for MFB and who also has contact, due to his profession, with chemicals, contributing to the occupational predisposition thesis of this disease.
The research did not receive any financial support.
The authors do not have any conflict of interest.
Information about data and material may be requested by email to correspondence author.
DFRL is the main author and responsible for the manuscript. DFRL and VCN wrote the manuscript and selected images and articles. MEAM is pathologist with large experience in dermatology. MEAM made supervision of the pathological aspects and differentials. MEAM and SAM are responsible by clinical part and helped with photos. CCO is the main supervisor of the article, responsible by review of image, text and diagnosis. All authors approved the final version of this article.