Lichen sclerosus et atrophicus is a chronic inflammatory skin disease that typically affects prepubertal girls and peri- or post-menopausal women in genital and perineal areas. In some cases, it can also manifest as extragenital lesions. Extragenital Blaschkoid lesions have infrequently been reported. Here, we report a case of extragenital Blaschkoid lichen sclerosus et atrophicus in a child.
Lichen sclerosus et atrophicus, Lichenoid eruptions, Dermatology, Blaschkoid, Pediatrics
Although lichen sclerosus et atrophicus (LSA) is a common inflammatory skin disease affecting the genital and perineal areas in prepubertal girls and peri- or post-menopausal women, extragenital lesions are less common, especially in a Blaschkoid distribution. Here, we present the case of a 12-year-old girl with extragenital Blaschkoid LSA that responded to high-potency topical steroids and also provide a brief review of the disease.
A 12-year-old African-American girl presented with a pruritic rash on her left back and axilla. This was diagnosed clinically as lichen simplex chronicus about 2 years prior when there was a single, scaly, lichenified plaque in her left inferior axilla. However, the patient had been lost to follow-up, and her rash continued to spread. She denied any history of folliculitis, trauma, or burns to the area. She denied any associated myalgias, arthralgias, muscle weakness, difficulty with movement, or oral or genital involvement. Her family history was unremarkable.
On the left upper back extending onto the axilla and chest in a linear distribution, there were multiple well-defined, ovoid, atrophic plaques with mottled hyperpigmentation and hypopigmentation (Figure 1). No induration or erythema was appreciated. Her shoulder had normal range of motion. A biopsy showed compact orthokeratosis, loss of the normal rete ridge pattern, papillary dermal edema, and a sparse lymphocytic infiltrate (Figure 2). Clinical pathologic correlation was consistent with Blaschkoid LSA.
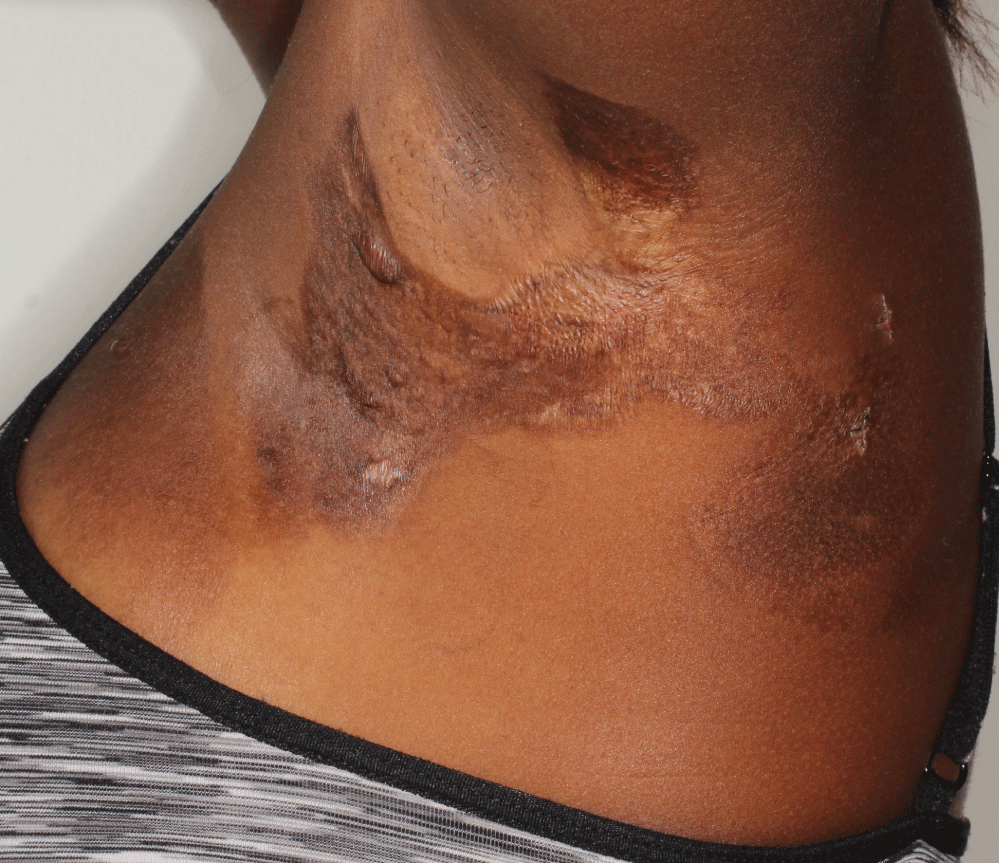 Figure 1: Well-defined, ovoid, atrophic plaques with mottled hyperpigmentation and hypopigmentation. View Figure 1
Figure 1: Well-defined, ovoid, atrophic plaques with mottled hyperpigmentation and hypopigmentation. View Figure 1
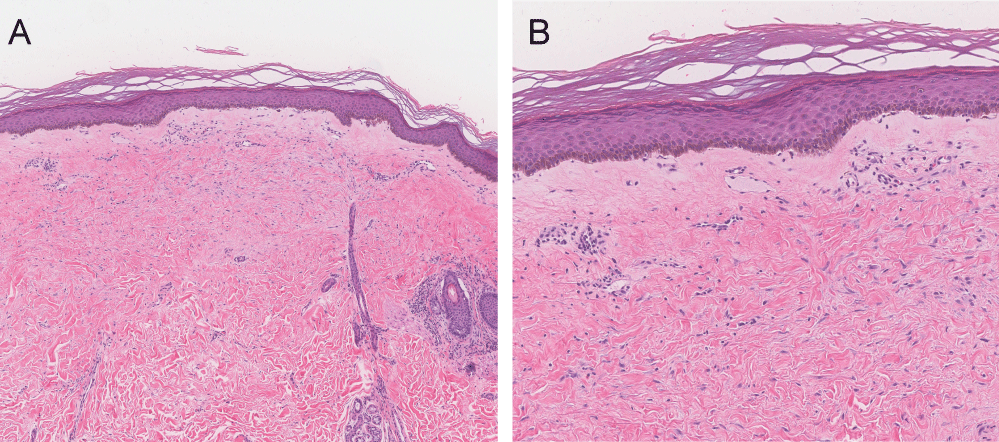 Figure 2: Compact orthokeratosis, loss of the normal rete ridge pattern, papillary dermal edema, and a sparse lymphocytic infiltrate A) H&E, original magnification x4; B) H&E, original magnification x10. View Figure 2
Figure 2: Compact orthokeratosis, loss of the normal rete ridge pattern, papillary dermal edema, and a sparse lymphocytic infiltrate A) H&E, original magnification x4; B) H&E, original magnification x10. View Figure 2
The patient was treated with a regimen of clobetasol ointment alternating with tacrolimus ointment. Mild symptomatic improvement was noted after one month of treatment.
LSA is a chronic inflammatory disease that typically affects the genital and perineal areas, with extragenital spread occurring in 15-20% of patients [1]. Prepubertal girls and peri- or post-menopausal women have the highest prevalence. While the etiology remains unclear, numerous hormonal and immunologic agents in addition to genetic predisposition have been implicated as pathologic factors. Nearly 75% of women with LSA were found to have increased antibodies against extracellular matrix protein 1, a significant building block of the dermis, thus supporting an autoimmune process [2]. However, this antibody is not routinely checked in patients, and it was not checked in ours.
The lesions of LSA usually present as ivory-colored and scar-like polygonal papules and plaques. Involvement of vulvar and perineal regions can produce a classic figure-of-eight configuration. Symptoms include vulvar pruritus, dyspareunia, and dysuria. However, extragenital lesions are usually asymptomatic and can typically be found on the neck, trunk, and proximal extremities. In rare cases, a Blaschkoid pattern can been identified as in our case [3]. This is thought to be a product of genetic mosaicism, where two distinct cell clones are present. The abnormal clone is ultimately stimulated by an environmental or immunologic factor, leading to its unmasking and the generation of segmental lesions.
LSA can be diagnosed clinically, but a biopsy is required for atypical cases, including extragenital lesions that can mimic other disease processes. LSA characteristically demonstrates an atrophic epidermis and a lichenoid infiltrate at the dermal-epidermal junction with loss of the normal rete ridges. In early lesions, papillary edema is found along with homogenization of collagen leading to eventual sclerosis. Scattered histiocytes, plasma cells, and mast cells can be present [4].
LSA is a progressive disease that can be disfiguring. Early treatment should include high-potency topical corticosteroids. Because of the side effects from long-term use of topical corticosteroids, calcineurin inhibitors can be utilized for continued management. Among other treatment options with limited efficacy, phototherapy and oral retinoids have been shown to be helpful. Cryotherapy, carbon dioxide laser, and focused ultrasound have also been used with varying rates of success [5].
All authors have contributed equally to this work.
None.
None.