Cutaneous manifestations of diabetes mellitus (DM) are a group of skin disorders that characteristically arise from prolonged chronic hyperglycemia and impaired insulin signaling or insulin resistance. There are several skin diabetic pathologies associated with the two main types of diabetes-type 1 (T1DM) and type 2 diabetes (T2DM), which tend to negatively impact the patients quality of life. The literature reveals variations in the skin barrier changes between the two DM types, and this may probably be a crucial factor for certain skin manifestations to be more common in one DM type over the other. However, despite this, the underlying pathophysiology affecting the skin in DM and the associated skin barrier changes between the two DM types is less understood and inadequately explored. The pathological mechanisms of several cutaneous manifestations in DM are still unclear, suggesting a complex interplay of various etiological factors that necessitate further exploration. The correction of raised blood glucose alone does not resolve the cutaneous manifestations in DM, hence involvement of a dermatologist at an early stage while managing DM patients can prove beneficial.
DM: Diabetes Mellitus; FFA: Free Fatty Acid; HbA1c: Glycated Haemoglobin; T1DM: Type 1 Diabetes Mellitus; T2DM: Type 2 Diabetes Mellitus; TEWL: Transepidermal Water Loss; QoL: Quality of Life
The skin mirrors the underlying pathology in diabetes mellitus (DM) [1]. The transepidermal water loss (TEWL), skin pH and lipid matrix of the stratum corneum (SC) are major determinants that characterise the skin barrier function [2,3]. Altered composition and activity of these determinants lead to skin barrier dysfunctions in DM and subsequent cutaneous manifestations such as xerotic skin, pruritus and cutaneous infections [4,5]. Thus, the cutaneous barrier function has become an area of increasing interest and study in DM.
TEWL is a marker of water diffusion from the dermis to skin surface through the epidermis (SC) [6]. A high TEWL is indicative of perturbed skin barrier leading to xerotic skin, whereas a low TEWL implies skin barrier disruption due to loss of ability of the SC to evaporate water [7,8]. Thus, a normal balance of TEWL is essential to regulate water content and maintain a healthy defense barrier.
To assess the TEWL in DM patients, Seirafi, et al. conducted an age-sex-matched case-control study on 34 type 2 DM (T2DM) patients [9]. The TEWL of different anatomical locations of the skin such as volar surface of forearm, extensor surface of the lower leg and forehead were measured using a noninvasive instrument called Tewameter® [9]. The authors observed no statistically significant differences betweenT2DM patients and controls (P > 0.05) (Table 1) [9].
Table 1: TEWL measured in various anatomical skin sites in Seirafi's study. View Table 1
In contrast, the newer age-sex-matched case-control studies performed by Han, et al. and Kim, et al. on 42 and 52 T2DM patients respectively, demonstrated a statistically significant reduced TEWL as compared to controls (Figure 1) [10,11]. Both studies used a Tewameter® to measure TEWL from various anatomical sites such as forearm, shin, mid-palm and sole along with volar side of the third finger and first toe [10,11]. All three studies had shortcomings in relation to the limited sample size which make the findings difficult to generalize and establish a cause-effect relationship. Regardless, the inconsistency in the results obtained from the older and newer studies make it important to analyse the potential cause for different outcomes.
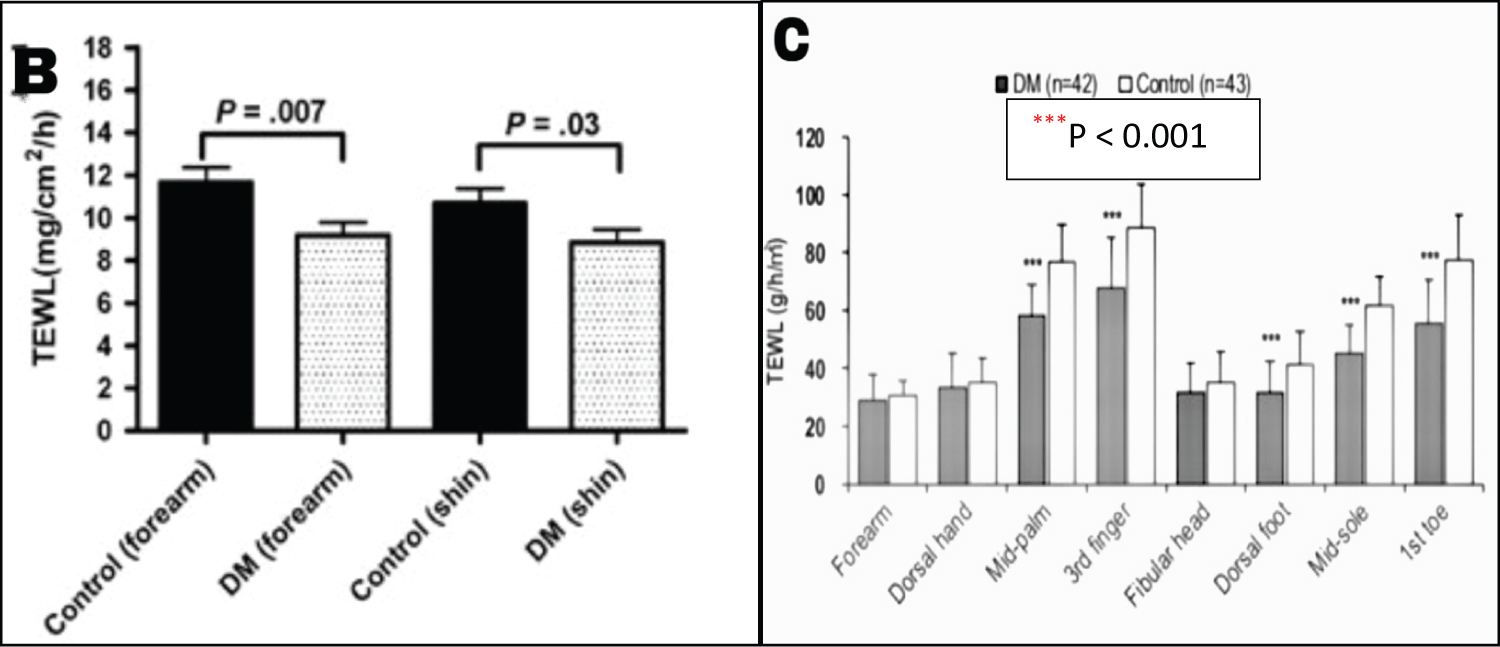 Figure 1: The TEWL measured in various anatomical sites in Kim's and Han's studies shows statistically significant differences between the T2DM patients and controls. Figure 1B: The diabetic group demonstrated significantly lower TEWL values on the forearm (P = 0.007) and shin (P = 0.03). Figure 1C: The diabetic group demonstrated significantly lower TEWL on the mid-palm, volar side of the third finger, dorsal foot, mid-sole and volar side of first toe (***P < 0.001). [10,11].
View Figure 1
Figure 1: The TEWL measured in various anatomical sites in Kim's and Han's studies shows statistically significant differences between the T2DM patients and controls. Figure 1B: The diabetic group demonstrated significantly lower TEWL values on the forearm (P = 0.007) and shin (P = 0.03). Figure 1C: The diabetic group demonstrated significantly lower TEWL on the mid-palm, volar side of the third finger, dorsal foot, mid-sole and volar side of first toe (***P < 0.001). [10,11].
View Figure 1
A plausible explanation to the difference in outcomes may have arisen from the different diagnostic inclusion criteria of DM. Seirafi's study included diabetics with HbA1c > 5.7%, while Han's and Kim's studies included diabetics with HbA1c > 6% and > 7%, respectively [9-11]. This implies that patients in the latter groups had poorer glycemic control as compared to the former group. From this understanding, it can be hypothesized that poorer glycemic control may significantly affect the skin barrier resulting in reduced TEWL, as seen in T2DM patients. Furthermore, as TEWL varies with different anatomical sites, the season, race/ethnicity and skin type, all these factors may have also contributed to the varied outcome [12]. The studies measured TEWL from different anatomical skin sites and Seirafi's study was conducted in Iran, while Kim's and Han's studies were performed in Korea, so the ethnicity of patients may have also influenced the rate of TEWL [9-11,13].
On the other hand, to evaluate the epidermal water evaporation rate (TEWL) in type 1 DM (T1DM), Okano, et al. performed an experimental study on STZ-induced T1DM mouse model [14]. The TEWL was evaluated from the dorsal skin of the mice using a vapos can instrument [14]. Prior to measuring the TEWL, a tape stripping technique was performed on the testing skin site in order to adequately remove the SC corneocytes to assess the epidermal function [14]. The authors demonstrated significant increase in TEWL in the diabetic mice as compared to control mice p < 0.01 (Figure 2) [14]. Furthermore, the TEWL returned to normal following insulin injections in diabetic mice [14]. This emphasizes the fact that a high blood glucose environment is unfavorable for an effective skin barrier [14,15]. However, this can be mitigated by insulin injections to restore the skin barrier functions in diabetics, as seen in Figure 2 [14].
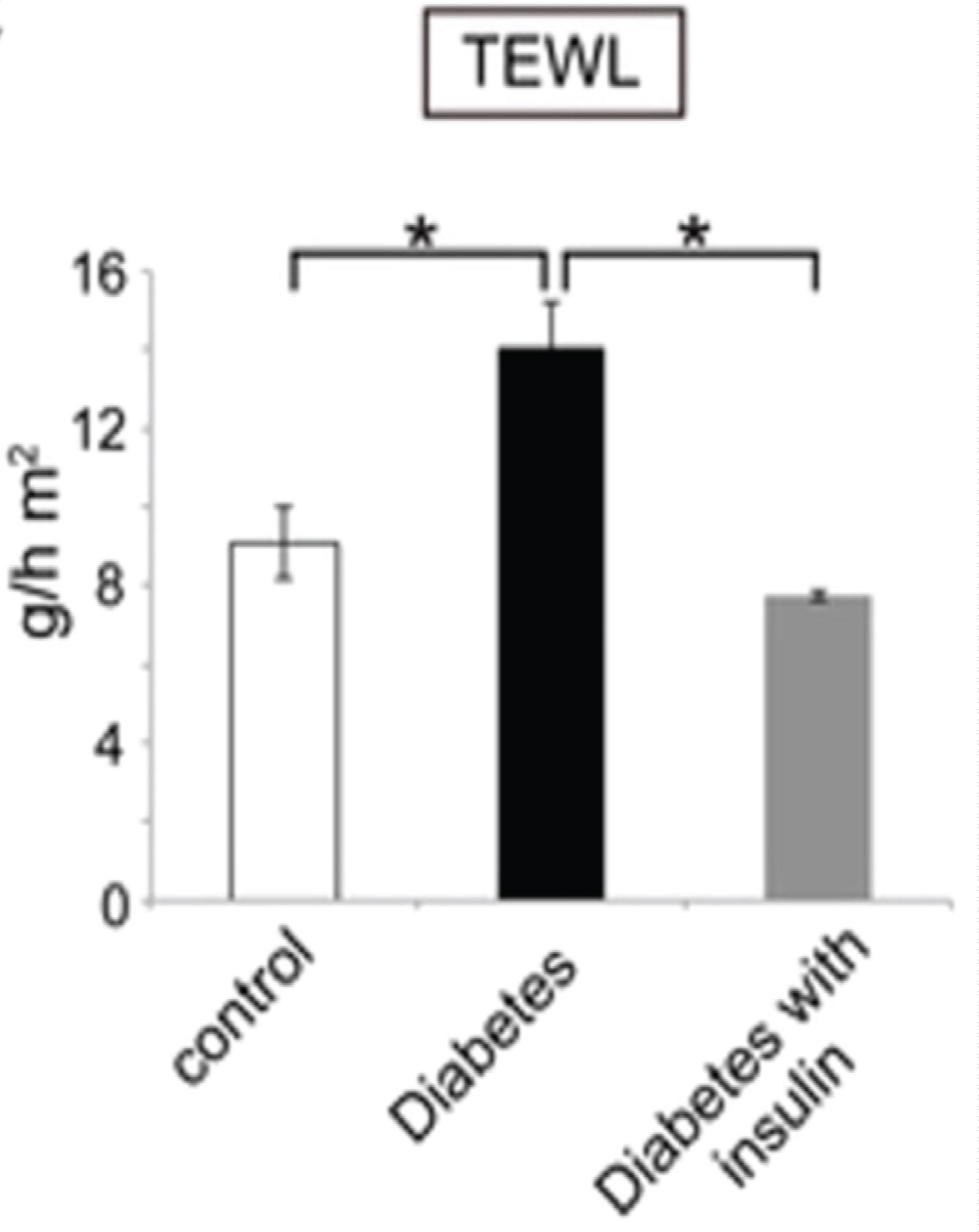 Figure 2: TEWL measured in control mice vs. diabetic mice. The transepidermal water loss after tape stripping is shown for the control mice (white bar), the diabetic mice (black bar), and insulin-treated diabetic mice (gray bar). TEWL is increased in T1DM mice as compared to control (15 g/h m2 vs. 9 g/h m2) *p < 0.01. This increased TEWL was mitigated with insulin treatment *p < 0.01 [14].
View Figure 2
Figure 2: TEWL measured in control mice vs. diabetic mice. The transepidermal water loss after tape stripping is shown for the control mice (white bar), the diabetic mice (black bar), and insulin-treated diabetic mice (gray bar). TEWL is increased in T1DM mice as compared to control (15 g/h m2 vs. 9 g/h m2) *p < 0.01. This increased TEWL was mitigated with insulin treatment *p < 0.01 [14].
View Figure 2
A limitation of the study was the tape stripping technique carried out 4 times using a scotch tape prior to TEWL measurement [14]. The repeated stripping, may have also contributed to the skin barrier disruption and subsequent increase in TEWL. Nevertheless, an increased TEWL causes xerotic skin and persistent itchiness which is frequently observed in T1DM patients [5,16].
The pH of normal skin surface is 4.1-5.8 [17]. The acidic pH of skin is integral to maintaining skin barrier function, SC lipid synthesis, epidermal differentiation and desquamation [17,18]. Thus, any deviations of skin pH; either too high or too low can have a negative impact on the skin barrier [17].
To assess the skin surface pH in T1DM patients, Mackiewicz-Wysocka, et al. conducted a case-control study in 105 T1DM patients and 53 age-sex-matched control cohort [19]. The skin pH was measured at three different sites (cheek, dorsal surface of forearm and foot) using the skin-pH-Meter® [19]. They found statistically significant differences between the two groups; the skin pH was lower, more acidic in T1DM compared to control in all regions measured: cheek (5.49 ± 0.42 vs. 5.69 ± 0.31; p = 0.001), forearm (5.41 ± 0.46 vs. 5.73 ± 0.69; p = 0.004), foot (5.20 ± 0.53 vs. 5.41 ± 0.41; p = 0.008) [19].
Furthermore, they observed an inverse relationship between HbA1c and skin pH in T1DM patients [19]. The T1DM patients with HbA1c ≥ 8% showed a significantly lower skin pH than those patients with HbA1c ≤ 8% having a higher skin pH [19].
Contradictory to this, Kim, et al., when using a T2DM db/db mice model, demonstrated that the skin pH was increased in type 2 diabetic mice as compared to control group (5.95 ± 0.02 vs. 5.68 ± 0.04, p < 0.0001) [11].
From these studies, it can be deduced that T1DM patients seem to have more acidic skin pH, while T2DM patients tend to have more alkaline skin pH. The imbalance in the "acid mantle" of the skin can lead to some consequences; such as the alkaline pH can make skin more susceptible to bacterial and fungal infections, as optimum antimicrobial activity is seen with acidic pH [17,20]. This concept is in line with the hypothesis made by several authors aboutT2DM patients being more susceptible to cutaneous infections [21-23].
The lipid matrix of the SC is extremely important to achieve epidermal homeostasis and maintain a competent skin barrier [2,24]. Therefore alterations in the SC lipid composition can derange the epidermal equilibrium and result in a perturbed skin barrier as seen in patients with DM [4,24].
The SC lipid composition has been studied in various diabetic animal models and DM patients.
Shetage, et al. performed a human volunteer study with 52T1DM patients and age-sex-matched controls to assess the composition of SC lipids [25]. Free fatty acids (FFAs) were found to be two-fold higher in diabetics (p < 0.05), while triglycerides (TG) were higher in the non-diabetic group (p < 0.05) [25].
The increased relative abundance of FFA and lower TG in T1DM was attributed to the action of lipase activity by micro-organisms that tend to be higher in diabetic skin [25]. The microbial lipase enhances the metabolism of TG, releasing FFA on skin surface [25]. Similarly, Sakai, et al. analysed the SC lipids in a T1DM mouse model and came to similar conclusions as the human study [26].
Building on the above, Yokota, et al. demonstrated that glycated epidermal skin samples in mice model showed an accelerated increase in FFA content; especially of the saturated fatty acid content in the epidermis [27]. Thus, a glycated epidermis seen in T1DM patients may have also increased the FFA composition.
On the other hand, besides analysing TEWL in DM patients, Kim, et al. also made another observation of the SC lipid composition in their previously mentioned study [11]. The authors conducted a matched case-control study on 52 T2DM patients (age > 40 years) and observed the main SC lipids (FFA, ceramides, cholesterol) to be reduced in T2DM patients to that of controls [11]. Age is a confounding variable that may have also influenced the outcome. Research has shown that advancing age can lead to significant reduction in SC composition [28,29]. Furthermore, the alkaline skin pH often seen in T2DM patients can also reduce enzyme activity of ceramide metabolism (b-glucocerebrosidase, acidic sphingo-myelinase), this in turn decreases the SC lipid composition [17].
However, a question that arises here is that glycated epidermis is common in both T1DM and T2DM, so the outcome of increased FFA should have been predicted in both conditions instead of T1DM alone.
To understand this, Yamane, et al. conducted an experimental study on T2DM rat models fed on a high fat diet (HFD) to closely resemble T2DM profile [30]. They found HFD reduced SC lipid composition by decreasing adiponectin levels [30]. The adiponectin released by adipose tissue is said to have anti-inflammatory and insulin-sensitizing properties that can reduce the risk of T2DM [31]. Additionally, it is thought to improve glucose and lipid metabolism [31]. Thus, the reduced adiponectin levels that arise from a long-term HFD impair glucose and lipid metabolism, often seen in T2DM [30,31].
Thus, the above-mentioned studies suggest that the reduced SC lipid composition in T2DM may have arisen from an elevated skin pH and a high fat diet and the latter's role in reducing adiponectin levels which in turn can affect lipid metabolism.
Epidermal turnover is the continuous process of formation of new keratinocytes in the basal layer of the epidermis and its eventual shedding as corneocytes from the skin surface [32]. The process of cell proliferation and the time taken for the epidermal turnover (normally 28-30 days) is reduced in DM [33,34]. The chronic hyperglycemia and deficient insulin signaling pathways result in reduced replication of skin cells, eventually leading to a thinner epidermis [14,27,35-37].
To this aim, Kadic, et al. performed an experimental study on STZ-induced T1DM rat model to evaluate the epidermal thickness at 6 and 12 months after induction of diabetes [38]. The diabetic status was confirmed by measuring the plasma glucose from tail veins of rats [38]. The skin samples were taken from the plantar surface of both hind paws, stained with H&E and examined under the microscope [38]. The initial basal level of epidermal thickness was 74.72 ± 17.56 μm, after 6 months a 30% reduction was observed and in 12 months a further 40.2% epidermal thickness reduction was noticed in T1DM rats (p < 0.001), while no significant difference was noticed in the healthy control group (p < 0.05) [38].
The result from the above study mostly tallied with the study undertaken by Boric, et al. on both T1DM and T2DM rat models [39]. The experiment was carried out in a similar manner to Kadic's study and the epidermal thickness was measured from plantar surface of hind paws [38,39]. Interestingly, although epidermal thinning was observed in both rat models, it was found to be more pronounced in T1DM rats than T2DM rats (Figure 3) [39].
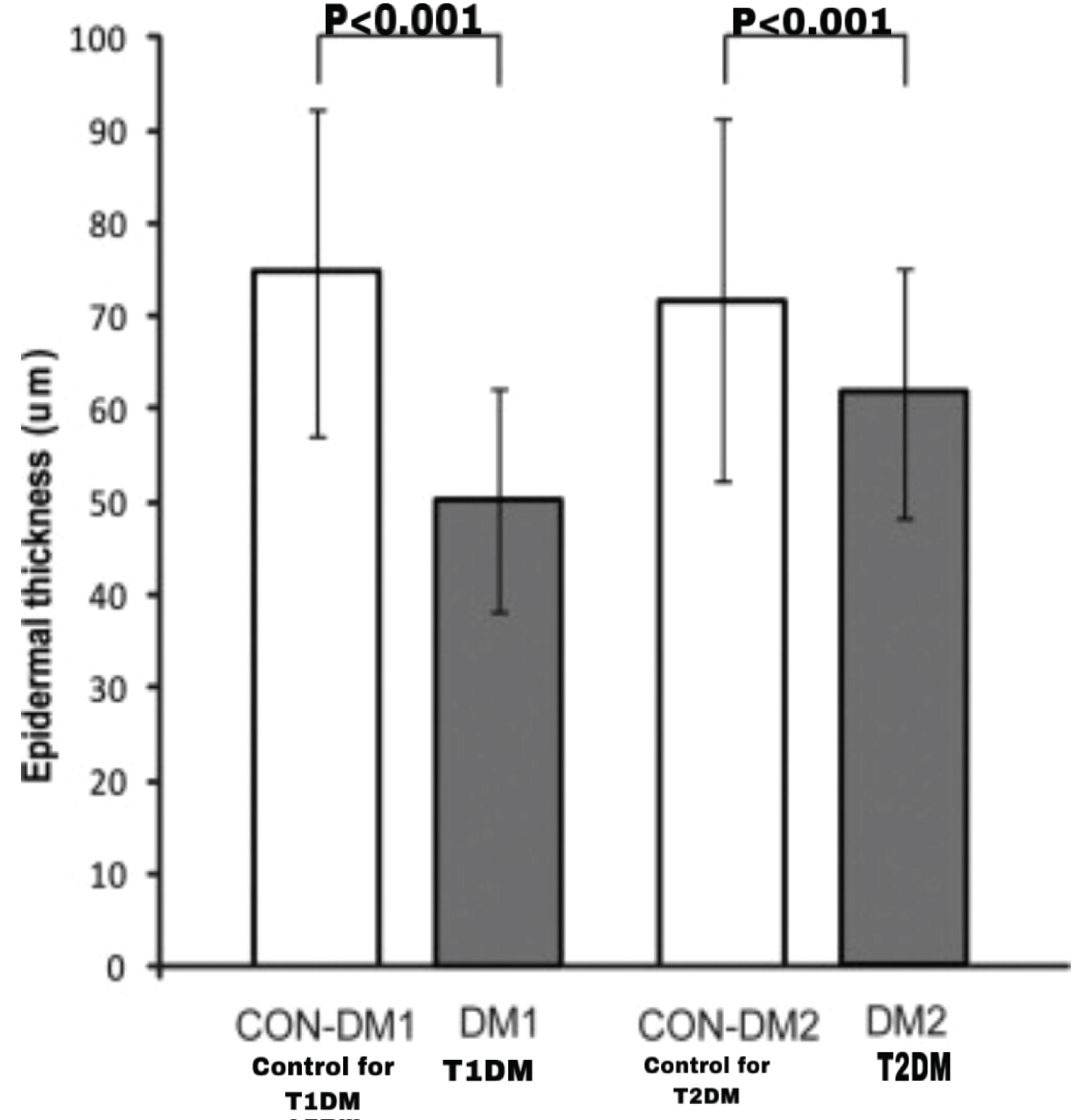 Figure 3: Epidermal thinning more pronounced in T1DM rats, compared to T2DM. The control group for T1DM rat model shows an epidermal thickness of 75 m, while T1DM rat models show a thickness of 50 m (p < 0.001). The control group for T2DM shows an epidermal thickness of 70 m, while T2DM rat model show a thickness of 62 m (p < 0.001) [39].
View Figure 3
Figure 3: Epidermal thinning more pronounced in T1DM rats, compared to T2DM. The control group for T1DM rat model shows an epidermal thickness of 75 m, while T1DM rat models show a thickness of 50 m (p < 0.001). The control group for T2DM shows an epidermal thickness of 70 m, while T2DM rat model show a thickness of 62 m (p < 0.001) [39].
View Figure 3
Skin manifestations are numerous and frequently observed in both T1DM and T2DM patients [40]. The prevalence of certain DM-associated cutaneous manifestations may be more common in one DM type over the other [40]. This may be due to the difference in the underlying pathophysiology (lack of insulin or insulin resistance) and the subsequent impact that it may have on the skin barrier and its determinants [41-43]. Additionally, the type of treatment modality such as skin complications from insulin injections or cutaneous side effects from oral hypoglycemic drugs can also increase the frequency of treatment-related cutaneous manifestations in DM patients [23].
Exploring this further, Sawatkar, et al. conducted a cross-sectional study on 500 T1DM patients [44]. The mean duration and age of onset in these patients were (4.4 ± 4.42 years and 12.5 ± 6.68 years) [44]. Around 67.8% of DM patients had one or more mucocutaneous involvement [44]. The authors reported skin-related complications of insulin therapy, such as lipohypertrophy as the commonest skin manifestation observed in 41% of T1DM patients [44]. It was noted that the longer the duration of DM, the more likely the associated complications from insulin therapy; lipohypertrophy [44]. A possible observer bias may have occurred as the investigators were aware of the diabetic status of the patients and their treatment modality at the time of study, which may have favoured insulin associated skin complications to be most common in T1DM and thus observed more closely. The bias could have been avoided by blinding the investigators and then assessing the outcome.
To investigate the commonest cutaneous manifestations in T2DM patients, Niaz, et al. performed a descriptive, cross-sectional study in Pakistan on 203 T2DM patients with associated skin manifestations [21]. The mean age and duration of diabetes were 50 ± 11 years and 8.5 ± 7 years [21]. Most patients in the study had unsatisfactory glycemic control [21]. They found cutaneous bacterial infections to be most common in T2DM patients, followed by cutaneous fungal infections and AN [21].
In contrast, a cross-sectional study done by Phulari, et al. in India on 200 T2DM patients, found cutaneous fungal infections to be most common followed by cutaneous bacterial infections [45]. Both these studies were done in South Asia where a hot, humid climate predominates, which may have led to the greater prevalence of cutaneous infections. The poor glycemic control may have also contributed to the above skin findings [46,47].
Many diseases and dermatological conditions can have a negative impact on patients' emotional well-being, their social environment and personal relationships [48]. Not surprisingly, patients with DM are subjected to lifestyle changes; such as healthier eating habits, regular physical exercise, monitoring blood glucose and adapting to the therapeutic interventions. All of these changes may have a long-term impact on their quality of life.
To this aim, Liu, et al. performed a qualitative and quantitative assessment of stigma in patients with T1DM and T2DM through a routine survey questionnaire consisting of relevant questions associated with stigma [49]. Among the 12,000 patients recruited, around 5422 patients completed the survey [49]. The impact of diabetic stigma was more prominent in T1DM than T2DM patients (76% vs. 52%, respectively, p < 0.0001) [49]. Interestingly in both groups, the perception of diabetes stigma increased with greater therapy intensity (insulin therapy) [49]. This could be due to the discomfort that may occur when injecting insulin on regular basis in public or at work places.
Building on the above, Bhardwaj, et al. conducted a comparative study on 120 adult patients with DM (n = 60 T1DM, n = 60 T2DM) to assess their level of QoL using a standardized questionnaire scoring tool [50]. The study found patients with T1DM (73.3%) to have a 'fair QoL' whilst majority of T2DM patients (46.7%) reported to have'poor QoL' [50]. Although this study hypothesized that QoL is better in T1DM patients, larger studies would be needed to confirm this theory.
Naughton, et al. reported similar results in their longitudinal observational study amongst the youth (aged 10-22 years) with DM [51]. The health related QoL was higher in patients with T1DM than T2DM [51]. The unequal sample size (n = 910 T1DM, n = 241 T2DM) may have favored the outcome to be better in T1DM in this study [51]. However, the probable reasons for the overall outcome in both studies to be in favor of T1DM may be because individuals with T2DM may hold accountable their sedentary lifestyle for their diabetic status adding to the stress, shame and anxiety. Moreover, the lifestyle changes they are subjected to may be difficult for everyone to follow.
Patients with DM are affected with a myriad of dermatological manifestations [16,21,45,52-59]. Despite this, studies have not explored the QoL in patients with DM-associated skin disorders. Therefore, future studies understanding the impact on the QoL on this group of patients would create more awareness both amongst physicians and dermatologists to ensure early intervention and better care.
The conclusions from the above-mentioned studies are summarised in the Table 2.
Table 2: Comparison between T1DM and T2DM. The various skin barrier determinants such as TEWL, skin pH, SC lipids and epidermal thinning are assessed between the two DM types along with QoL and common DM-associated skin manifestations. View Table 2