American College of Rheumatology recommends Bisphosphonates as the first choice drug for Glucocorticoids Induced Osteoporosis (GIOP). But denosumab is the promising drug that is recommended as the first choice for post-menopausal osteoporosis. This study was conducted to light up the efficacy and safety of denosumab compared to bisphosphonates in GIOP.
Randomized Controlled Trials (RCTs), retrospective, prospective observational Studies, conference abstracts were searched in PubMed, Web of Science, Embase, China National Knowledge Infrastructure (CNKI), clinicaltrials.gov, WHO clinical trials registry, Cochrane library. Studies analyzing the use of denosumab for GIOP were identified and reviewed independently by 2 authors for inclusion. A random-effect model was used for meta-analysis and was conducted using Review Manager 5.3.
12 studies with 1562 patients were identified for qualitative analysis. 5 (out of 12) studies were included for meta-analysis to compare efficacy and safety of denosumab to bisphosphonates. There was no statistically significant difference between denosumab and bisphosphonates groups for the fracture risk: risk ratio (RR) = 0.62, 95% Confidence interval (CI) = [0.19, 1.99]; adverse events: RR = 1.67, 95%CI = [0.75, 3.75] and Change in femoral neck Bone mineral density (BMD): weighted mean difference (MD) = 1.02 95%CI = [-0.76, 2.80]. But there was statistically significant difference in change in total hip BMD and lumbar spine BMD: MD = 1.03 95%CI = [0.12, 1.94] and MD = 2.48 95%CI = [1.74, 3.23] respectively.
This study suggested that there was no significant difference in adverse events and fracture risk between denosumab and bisphosphonates but there was a rise in BMD in total hip and lumbar spine in the denosumab group. Thus, long-term follow-up might report fracture risk-benefit in favor of denosumab in GIOP. More long-term follow-up RCTs are required to crystallize the safety and complications of denosumab.
Glucocorticoid, Osteoporosis, Denosumab, Bisphosphonates, Meta-analysis, Secondary osteoporosis
Glucocorticoids (GC) are one of the important drugs that are widely used for inflammatory and autoimmune diseases. But it comes with the price of many side effects on various organ systems. One of the side effects being osteoporosis, in fact Glucocorticoids Induced Osteoporosis (GIOP) is the most common cause for secondary osteoporosis [1]. In patients taking long term GC, up to 10% are diagnosed with clinical fractures and up to 40% have radiographic evidence of vertebral fracture [2,3]. And also, for the same BMD risk of fracture is higher in GIOP than postmenopausal osteoporosis [4]. Locascio, et al. reported that there is a loss of up to 12% BMD during the first few months of initiating corticosteroids and after continue to decrease up to 4% annually [5]. Despite all these evidences only a few receive preventive therapy for GIOP and many receive treatment only after fracture [6].
Bisphosphonates are recommended as the first drug of choice for prevention and treatment of GIOP along with optimizing calcium, vitamin D and life style modifications as smoking cessation, limiting alcohol, weight bearing exercise [7]. But patients taking long term GC also include children, young men and premenopausal women. Safety of use of bisphosphonates in these groups of population is not well understood [8,9] and drug compliance with bisphosphonates is poor [10] which makes this study a necessary, to find out if denosumab can be the alternative for prevention and treatment of GIOP.
Denosumab is a fully human monoclonal (igG2) antibody which attaches to Receptor activator of nuclear factor-κB ligand (RANKL) inhibiting interaction with RANK on the osteoclast membrane thus blocking differentiation, activation and survival of osteoclast. This result decrease in resorption of bone to the formation of bone, leading an increase in BMD and reducing fracture risk. Several RCTs and studies have shown the efficacy and safety of denosumab compared to bisphosphonates in post-menopausal osteoporosis [11-14]. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice guidelines recommends the use of denosumab as the first line for treatment and prevention of postmenopausal osteoporosis [15]. But pathophysiology of post-menopausal osteoporosis and GIOP is different. In post-menopausal osteoporosis, there is increased resorption of bone via RANKL due to estrogen deficiency [16,17] whereas in GIOP there is not only increased resorption of bone due to GC but also there is antagonist effect of GC to Wnt signaling which causes inhibition of osteoblast differentiation [18,19]. GC also reduces absorption of calcium from the intestine and increase excretion from the kidney.
Due to above mentioned drawbacks of bisphosphonates in GIOP, established efficacy of denosumab to treat primary osteoporosis and also rapid reversibility of effect of denosumab after discontinuation [20] could make denosumab a better choice of drug for young population particularly in premenopausal women with childbearing potential. It has been approved to use in GIOP only in men and women with a) Multiple risk factors for fracture b) History of osteoporotic fracture c) Who have failed or are intolerant to other anti-osteoporotic treatments d) Who are either continuing or initiating ≥ 7.5 mg/day prednisolone or its equivalent and planned to use the drug for at least 6 months [21]. Thus, this study was conducted to analyze current knowledge on efficacy and safety of denosumab in GIOP.
This study was conducted according to Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement [22]. Search strategy, inclusion criteria, exclusion criteria, data extraction, method of bias assessment, statistical analysis were determined prior to the start of the study.
Electronic database of PubMed, Web of Science, Embase, China National Knowledge Infrastructure (CNKI), clinicaltrials.gov, WHO clinical trials registry, Cochrane library were searched from inception to January 25, 2019 using keywords and corresponding Mesh terms of "denosumab", denosumab bisphosphonate "glucocorticoid induced osteoporosis", "steroid induced osteoporosis", "glucocorticoid" and "osteoporosis". References of selected studies were manually searched for additional studies. All references were imported to EndNote X9.
Studies with following criteria were included 1) Population: Patients taking GC or diagnosed as GIOP 2) Intervention: Denosumab (at any dose and duration) used to treat or prevent GIOP given subcutaneously 3) Comparison: Comparison with bisphosphonates or placebo or baseline data 4) Outcomes: With any of following outcomes: Fracture risk, adverse events, change in bone turnover markers, change in BMD in total hip, lumbar spine, femoral neck, distal radius, greater trochanter 5) RCTs, retrospective, prospective observational Studies, conference abstracts 6) Written in any languages. Studies would be excluded if 1) Post-menopausal osteoporosis patients on steroids treated with denosumab 2) Cancer patients 3) GIOP treated with teriparatide or other medications than denosumab.
Based on the above criteria two authors independently screened for possible studies and classified as included and unclear. Unclear studies were discussed with the third author (SX) and were decided for inclusion. Studies were included based on study titles first then duplicates were removed using EndNote X9. Full abstracts were then read and studies were selected for full article reading.
Two authors independently extracted data from selected studies. Study name, study design, mean age, mean prednisolone dose during initiation of denosumab, mean prednisolone duration, intervention used and number of participants, co interventions, duration of study, outcomes of study were extracted. Outcomes on fracture risk, adverse events, change in BMD in total hip, lumbar spine, femoral neck, distal radius, greater trochanter were extracted quantitatively. Authors were not contacted for missing data.
Two authors independently assessed bias in included studies based on Cochrane collaboration's tool for assessing risk of bias [23]. The tools included were random sequence generation, allocation concealment, blinding and selective outcome reporting. Studies were considered with high risk of bias if randomization was absent, participants were not blinded to treatment and more than 2 included tools had high risk of bias. Unclear bias was considered if more than 2 included tools had unclear risk of bias.
Review manager 5.3 was used to conduct meta-analysis. Random effects model was used for obvious presence of between study heterogeneity. Dichotomous data were expressed as risk ratio (RR) with 95% confidence interval (CI). Continuous data were expressed as weighted mean difference (MD) with 95% CI. Funnel plot was used to assessed the publication bias. In Sensitivity analysis, meta-analysis was done removing each study at a time. Sub-group analysis was done on basis of risk of bias: high risk and unclear risk. Heterogeneity was evaluated using I2 tests.
After the search, 354 relevant articles were found and imported to EndNote version X9. After removing duplicates 267 articles were screened on basis of titles and abstracts, 23 articles were assessed for eligibility by full text reading and 11 were excluded as per predefined criteria.
Thus 12 articles were selected for qualitative analysis [24-35] and 5 were selected from them for meta-analysis [31-35] in which denosumab was compared to bisphosphonates with outcomes as our predefined inclusion criteria. Among twelve included studies four were RCTs [26,31,32,35], three were retrospective studies [28,29,33], three were prospective observational studies [24,25,34]. Among four conferences abstracts [27,30,33,34], two did not mention about study design [27,30]. No other studies were identified by manual search in reference lists of included studies. Figure 1 demonstrates the flowchart of details on study screening and selection.
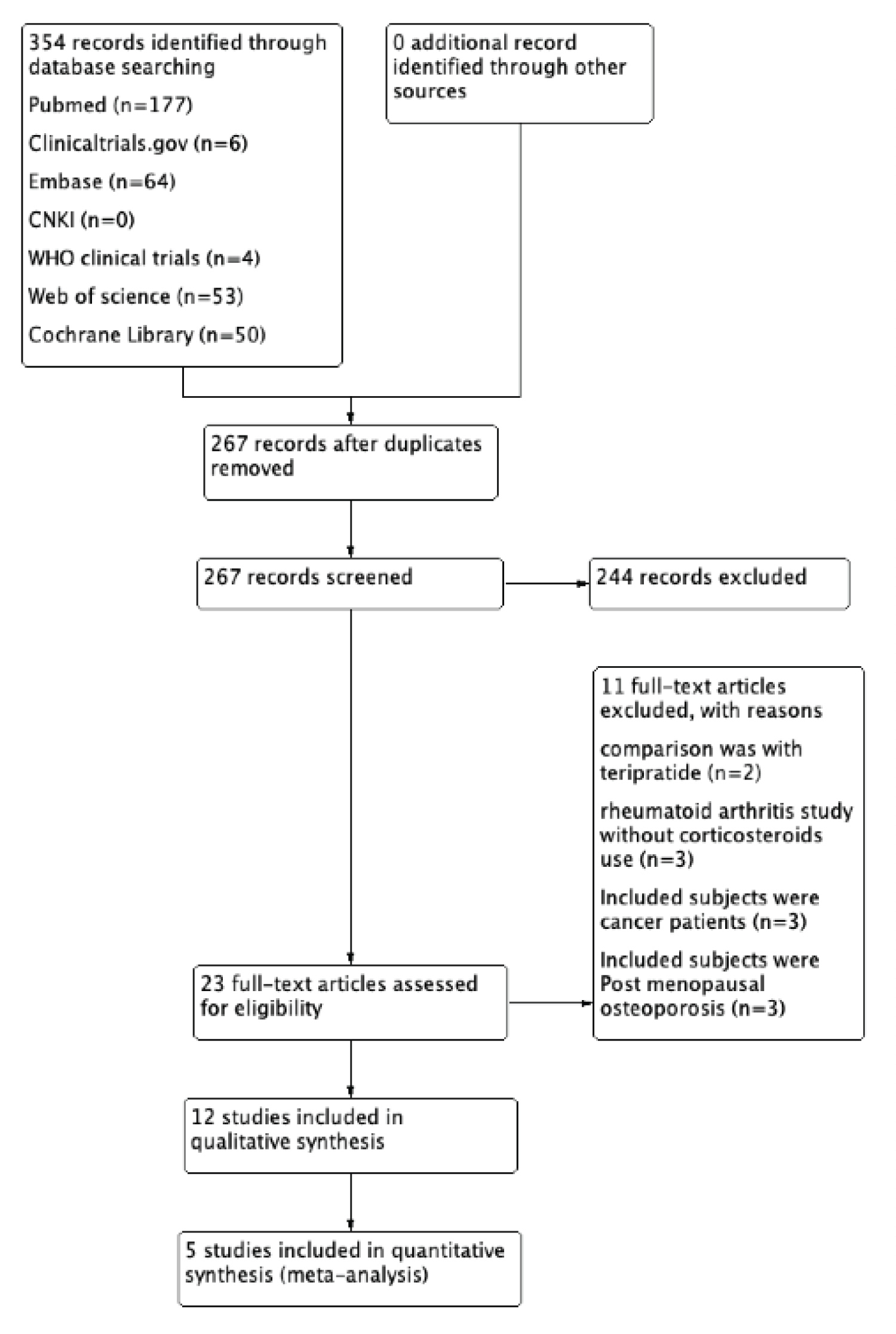 Figure 1: Flowchart of details on study screening and selection.
View Figure 1
Figure 1: Flowchart of details on study screening and selection.
View Figure 1
Among the included studies, nine of them were from Japan, one from the USA, one from Hong kong, and one was multicenter RCT. Publication year ranged from 2010 to 2018. In total it included 1562 GIOP patients taking 60 mg of denosumab subcutaneously (SC) every 6 months or 180 mg SC every 6 months (n = 33) [26]. An average dose of prednisolone during the start of treatment ranged from 3.59 mg/day to 14.45 mg/day in denosumab group and in comparison group it ranged from 4.12 mg/day to 13.35 mg/day. Cointerventions used in the different study were calcium and vitamin D (Alfacalcidol, Eldecalcitol, calcitriol). Underlying diseases that caused the use of prednisolone in different studies were mainly rheumatic diseases, ulcerative colitis, Crohn's diseases, asthma. Further information on general characteristics of included studies is presented in Table 1.
Table 1: General characteristics of included studies. View Table 1
Four of the included studies had a low risk of bias [26,31,32,35]. Two studies had a high risk of bias [25,28] and the rest had an unclear risk of bias. Summary of bias assessment is presented in Figure 2.
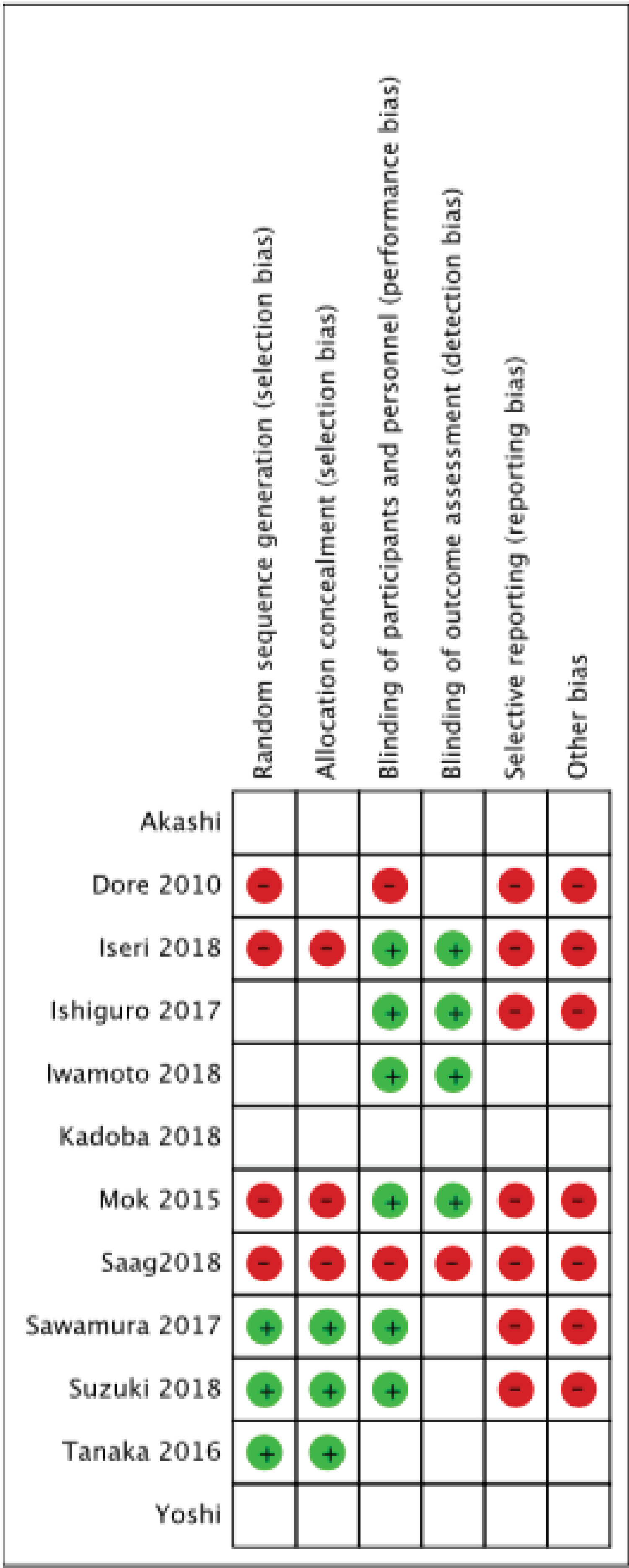 Figure 2: Risk of bias summary, - no bias, + bias, blank- unclear bias.
View Figure 2
Figure 2: Risk of bias summary, - no bias, + bias, blank- unclear bias.
View Figure 2
Eight studies included data on fracture risk [24-26,29,31,33-35]. Among studies reported an incidence of fracture, Dore, et al. reported a fracture of less than 2% and fracture risk was not correlated to the use of denosumab, bisphosphonates, glucocorticoids. Iwamoto, et al. reported 2 fragility fractures in the pelvic ring. Studies that were included in meta-analysis revealed no statistically significant difference in fracture risk between denosumab and bisphosphonates group risk ratio (RR) = 0.62, 95% Confidence interval (CI) = [0.19, 1.99] with heterogeneity I2 of 52% (Figure 3).
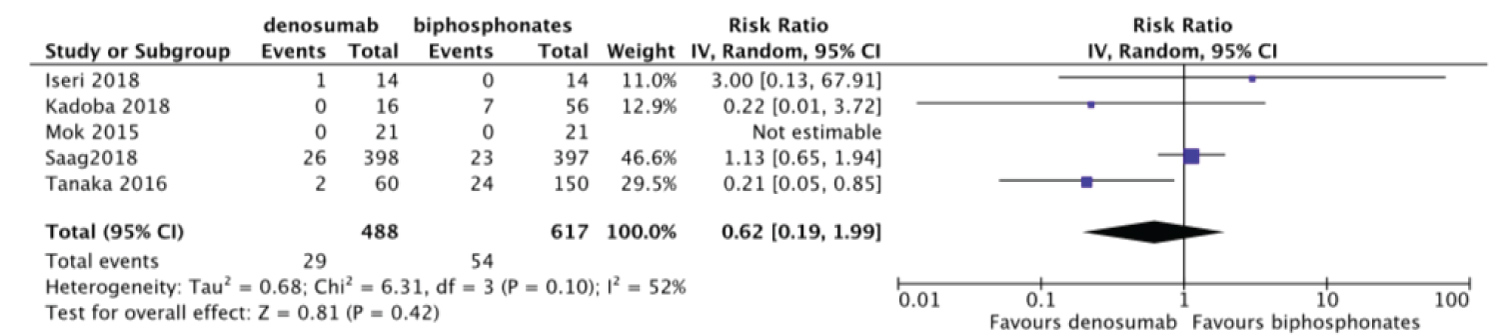 Figure 3: Forest plot of included studies comparing fracture risk.
View Figure 3
Figure 3: Forest plot of included studies comparing fracture risk.
View Figure 3
Mok, et al. reported no fracture incidence in both treatment arms thus removed from meta-analysis. In the outcome of fracture risk, we assessed the funnel plot to evaluate publication bias, which revealed no evidence of publication bias (Figure 4). We performed sensitivity analysis to determine if overall effect size changes leaving out one study at a time for the outcome of fracture risk. The result of sensitivity analysis showed the overall effect size was not changeable (Table 2). We further performed subgroup analysis on basis of risk of bias. The result of subgroup analysis revealed, studies with high/unclear risk of bias favor denosumab whereas studies with no high/unclear risk of bias favor bisphosphonates (Table 3).
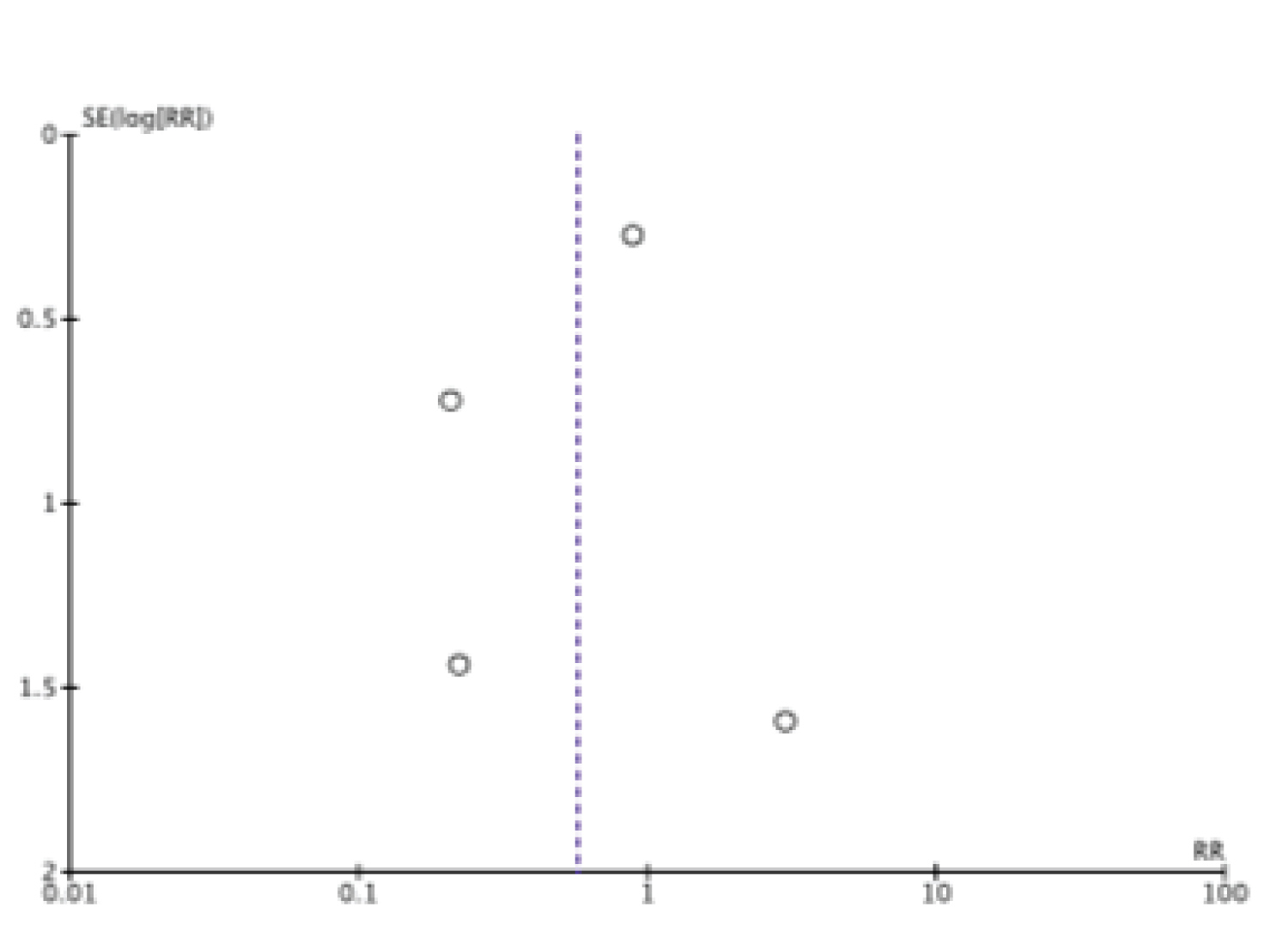 Figure 4: Funnel plot (fracture risk).
View Figure 4
Figure 4: Funnel plot (fracture risk).
View Figure 4
Table 2: Sensitivity analysis (fracture risk). View Table 2
Table 3: Subgroup analysis based on the risk of fracture. View Table 3
Denosumab being relatively new drug there exists a concern of long term safety and adverse effects. Effect of denosumab on healthy Chinese man was reported by Chen, et al. which concluded denosumab was well tolerated and there was no concern about its safety [36]. Large RCTs on postmenopausal osteoporosis has revealed the safety profile of denosumab [12,13]. It has been reported as a useful therapeutic option for patients with renal insufficiency [37]. It has been reported that denosumab also improves glycemic control on specific group [38] and is also effective in bone marrow edema syndrome [39]. But there are some contraindication and adverse effects in use of denosumab [40]. Also reproductive toxicity [41], Alopecia areata [42] has been associated with the use of denosumab. Adverse events that were reported in the included studies are presented in Table 4. Denosumab being an antibody against RANKL, major concern in the use of denosumab is the incidence of infection.
Table 4: Adverse events of denosumab in GIOP. View Table 4
a) Infection: Saag, et al. reported 5 cases with pneumonia in the denosumab group and 5 in risedronate group. The rate of infections between groups was similar. Mok, et al. reported 7 cases of minor upper respiratory infection which needed no antibiotics. Iseri, et al. reported a case of tuberculosis after the use of denosumab for 10 months. American College of Rheumatology (ACR) recommends denosumab as a conditional recommendation for GIOP due to lack of safety data in people treated with immunosuppressive agents. Three of the included studies reported data of adverse effects on concomitant use of immunosuppressive agents [26,29,31]. Iwamoto, et al. reported 4 out of 5 patients treated with immunosuppressive suffered from infections. Saag, et al. reported the incidence of infection was similar in patients with or without immunosuppressive agents (biologic or non-biologic). Dore, et al. reported the use of immunosuppressive agents in 8 patients and adverse events were comparable between denosumab and placebo groups. It has also been reported that the incidence of serious and opportunistic infections treated with denosumab and biologics for rheumatoid arthritis were low [43].
b) Eczema: Eczema resulted in withdrawal from a study for 1 patient but it got better without any treatment after withdrawal from study [32]. Other skin related adverse effects reported were skin rash and alopecia.
c) Hypocalcemia: Vitamin D and calcium use are essential during treatment with denosumab [44,45]. Calcium and vitamin D were used as cointervention in many studies included in this review (Table 1). About the incidence of hypocalcemia during the treatment Iseri, et al. reported 2 cases in the denosumab group, Kadoba, et al. reported 3 cases in denosumab and 6 cases in bisphosphonates group. Further hypocalcemia is a contraindication for denosumab use, calcium and vitamin D level should be checked regularly during the treatment period. Iseri, et al. and Saag, et al. excluded patients with low calcium and vitamin D level from their study.
d) Osteonecrosis of Jaw (ONJ): Bad dental hygiene, dental extraction, use of chemotherapy, dental prosthetics are risk factors to develop osteonecrosis of jaw [46]. Denosumab being a potent antiresorptive agent there is a possibility of incidence of ONJ. Kadoba, et al. reported 1 case each in denosumab and bisphosphonates group. 10 years extension of FREEDOM trails reported 13 cases of ONJ consistent with 5.2 per 10,000 participant-years [13].
Adverse events frequently reported with use of denosumab were e) Atypical fracture of femur f) Back pain g) Arthralgia. In the included studies other adverse events associated with denosumab use were cardiac failure, transient ischemic attack, hypertension, anti-denosumab antibodies [31] as presented in Table 4. Hepatic toxicity has also been reported with use of Denosumab [47].
We performed Meta-analysis comparing the incidence of adverse events between denosumab and bisphosphonates. There was no significant difference in the incidence of adverse events between denosumab and bisphosphonates RR = 1.67, 95%CI = [0.75, 3.75] with heterogeneity I2 of 75% (Figure 5).
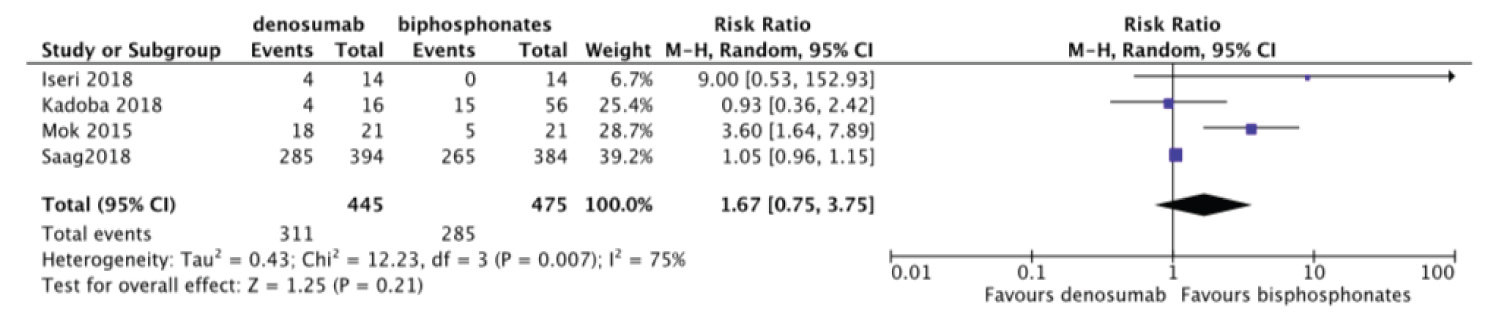 Figure 5: Forest plot of included studies comparing adverse events.
View Figure 5
Figure 5: Forest plot of included studies comparing adverse events.
View Figure 5
Current knowledge of denosumab confirms positive effect in increasing BMD [13,14]. Further, it has been reported to be superior to bisphosphonates [11] in treating post-menopausal osteoporosis. Iwamoto, et al., Mok, et al., Iseri, et al., Saag, et al. reported change in lumbar BMD by 4.4%, greater than 3%, 5.3%, 4.1% respectively. Change in BMD was higher in GC continuing group than GC starting group as reported by Saag, et al. which could be probably because of a higher dose of GC in GC starting group. Further, Saag, et al. reported denosumab not only to be non-inferior but superior to risedronate.
One of the studies reported the change in distal radius BMD but there was no significant change in BMD after use of denosumab for 12 months [32]. Another study where first 6 months patients were treated with either denosumab or bisphosphonates then changed to other agent showed those who were treated with bisphosphonates then changed to denosumab after 6 months had their BMD increased in lumbar spine, femur neck and greater trochanter compared with baseline. But switching from denosumab to bisphosphonates after 6 months decreased greater trochanter BMD but there was increased in BMD in other sites [30]. Iwamoto, et al. reported patients who transitioned from bisphosphonates showed an increment of 4.17% and from teriparatide showed an increment of 3.71% in overall BMD after 12 months [29]. Similarly, Suzuki, et al. reported 4.2% of increment in lumbar BMD and 4.7% of increment in total hip BMD in bisphosphonates pretreated group [28]. Iwamoto, et al. reported multivariate logistic analysis which revealed a dose of prednisolone and body weight were associated with the change in greater than 3% BMD.
Meta-analysis was done to compare the efficacy of denosumab to bisphosphonates in change in BMD. There was no statistically significant difference for change in femoral neck BMD MD = 1.02 95%CI = [-0.76, 2.80] I2 = 98% (Figure 6). Change in total hip and lumbar spine BMD were significant MD = 1.03 95%CI = [0.12, 1.94] I2 = 91% (Figure 7) and MD = 2.48 95%CI = [1.74, 3.23] I2 = 78% (Figure 8) respectively. Change in BMD in the distal radius and greater trochanter were not analyzed as it was reported in one study only.
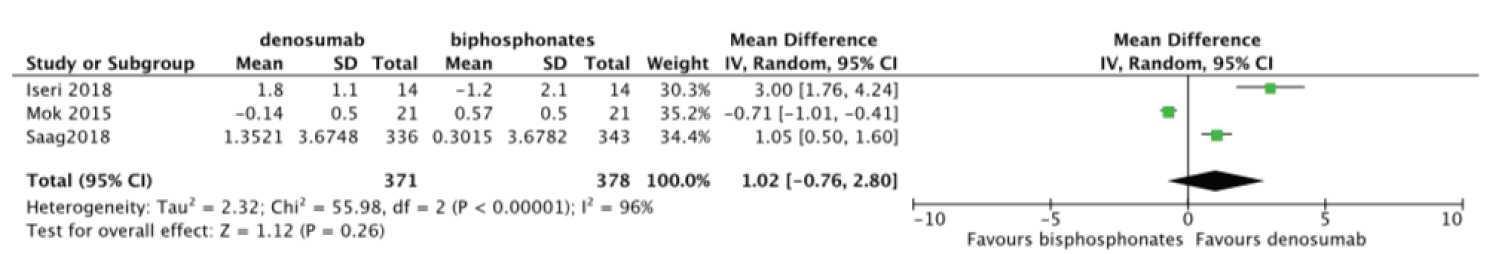 Figure 6: Forest plot of included studies comparing femoral neck BMD.
View Figure 6
Figure 6: Forest plot of included studies comparing femoral neck BMD.
View Figure 6
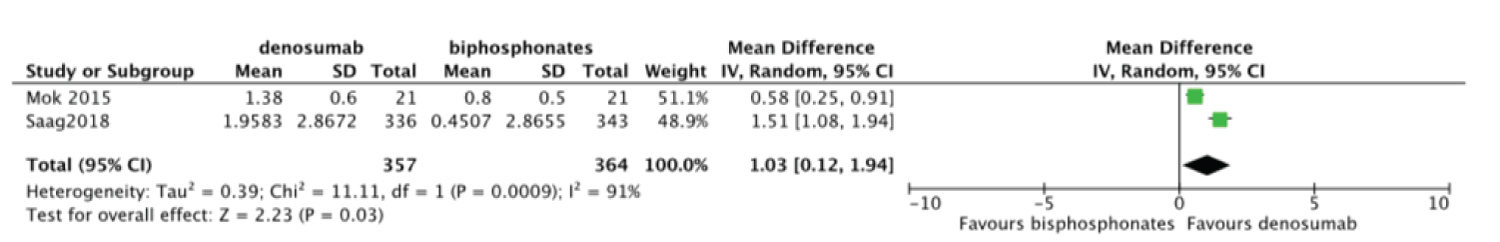 Figure 7: Forest plot of included studies comparing total hip BMD.
View Figure 7
Figure 7: Forest plot of included studies comparing total hip BMD.
View Figure 7
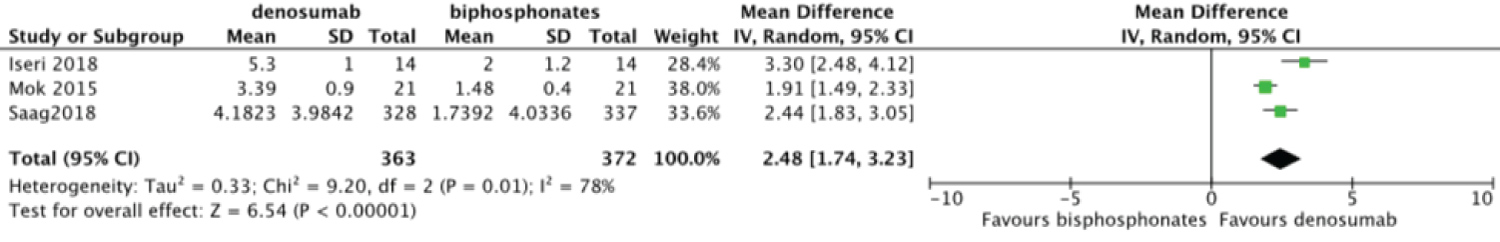 Figure 8: Forest plot of included studies comparing lumbar spine BMD.
View Figure 8
Figure 8: Forest plot of included studies comparing lumbar spine BMD.
View Figure 8
Seven studies reported the change in bone turnover markers [25,26,28,29,31,32,35]. Change in level of procollagen type 1 N-terminal propeptide (P1NP), osteocalcin, bone alkaline phosphatase (BAP) were measured as bone formation markers, whereas tartrate-resistant acid phosphatase-5b, type I collagen cross-linked C-telopeptide (CTX) were measured as markers of bone resorption. Use of denosumab resulted in a rapid decrease in bone turnover markers in included studies. Mok, et al. reported marked decreased in bone turnover markers in patients that were switched from bisphosphonates to denosumab than who continued bisphosphonates at 12 months. Suzuki, et al. and Iseri, et al. reported marked decrease in both denosumab and bisphosphonates groups, but between these groups there was no significant difference. Saag, et al. reported marked reduced in CTX in the 10th day of denosumab which makes it a better option for GIOP as GC induce rapid loss of BMD in the first few months. Dore, et al. reported baseline P1NP correlated with BMD increment for lumbar spine and total hip. They also reported CTX and P1NP levels increased at the end of each 6 months dosing interval pointing requirement of next dose.
Our systemic review and meta-analysis reviewed all available studies on the efficacy and safety of denosumab in GIOP. We found that denosumab increased BMD in different bone sites, decreased bone turnover markers but there was no significant difference in fracture risk and adverse events when compared with bisphosphonates. Although there was a gain in BMD, which didn't correlate with fracture risk benefit. This could be because of the short follow up period in the included studies or these points out multifactorial association with fracture incidence [48]. Our findings were similar to meta-analysis done on post-menopausal osteoporosis for the efficacy and safety of denosumab compared with bisphosphonates [14].
There were only 1 large RCT and 2 small RCTs available on this topic. To our knowledge, this is the first meta-analysis done on this topic which quantifies all possible outcomes data available in the literature. To increase comprehensiveness and to balance the picture on the efficacy of denosumab we included grey literature too, which also helped to reduce publication bias. We included 2 conference abstracts in meta-analysis. Thus, we did a subgroup analysis based on a risk of bias for fracture risk, which resulted studies with high/unclear risk of bias favor denosumab whereas studies with no high/unclear risk of bias favor bisphosphonates. There was significant heterogeneity in this meta-analysis which could be because there were few studies in this meta-analysis and also the fact we considered including RCTs and observational studies together [49]. Although there is no meta-analysis on this topic, a systemic review has been published [50]. Coskun reported RCTs and observational studies but didn't include conference abstracts. Coskun also added 2 studies which included post-menopausal osteoporosis patients on GC therapy which we didn't include in our study [51,52].
Dore, et al. recruited 29 patients who took both denosumab and bisphosphonates which give us insight on how the outcome would be after switching from bisphosphonates to denosumab because bisphosphonates effect persists after discontinuation too. They reported a marked decrease in bone turnover markers and had greater increased in BMD. Switching from one osteoporosis agent to denosumab was made in studies conducted by Ishiguro, et al. [24], Suzuki, et al. [28], Iwamoto, et al. [29], Mok, et al. [35], Iseri, et al. [32], all of these studies reported continuous increased in BMD after switching.
ACR recommends denosumab as a conditional recommendation for GIOP due to lack of data in concomitant use with immunosuppressants. In our study, we found out that there was no significant difference in adverse events between denosumab and bisphosphonates groups. Further, studies that included concomitant use of immunosuppressants also reported no significant difference in adverse events. A major concern with denosumab is an infection, as immune cells also express RANK. But some studies suggested denosumab has no effect on RANKL of immune system [53,54] suggesting it to be safe in terms of infection incidence. ACR also recommends teriparatide as a conditional recommendation for GIOP but to our knowledge, there is no available head to head trials comparing denosumab and teriparatide in GIOP. ACR first choice for GIOP, bisphosphonates might not be ideal for women with child bearing potential as bisphosphonates have a strong affinity to the bone and stay in bone for years even after discontinuation, this might be a concern in young female population who commonly have an autoimmune disease requiring long term GC.
Further, for real world adherence of drugs, not only efficacy and safety but cost effectiveness of drugs also should be evaluated. We searched for studies which compared denosumab with other available options for osteoporosis in terms of cost effectiveness. Hiligsmann, et al. and Yoshizawa, et al. reported denosumab to be cost effective than other options for osteoporosis [55,56]. Study on drug compliance also reported denosumab has higher compliance [57].
Our study had limitations, the number of studies were few and there was only 1 large RCT. Duration of follow-up in all included studies was short thus change in BMD didn't correlate with fracture risk benefits and long-term adverse effects were underestimated. Most of the studies were carried out in Asia so it might be hard to estimate safety and tolerability in other groups of the population. Multicenter large RCT by Saag, et al. was funded by a pharmaceutical company which might increase performance bias. None of the included studies reported using zoledronic acid which is one of the most potent bisphosphonates. But it is worth to mention that in post-menopausal osteoporosis denosumab was found more effective in increasing BMD and decreasing bone turnover markers than zoledronic acid [58].
As per currently available evidence, denosumab is effective in increasing BMD with no difference in adverse events when compared to bisphosphonates. Although denosumab has some limitations, it also has benefits of cost effectiveness and drug adherence when compared to bisphosphonates. But only a few studies are currently available thus more large RCT with long term follow up and with the primary outcome as fracture are needed. Also, studies assessing the safety of denosumab in women of childbearing potential and fracture risk in GC treated children are needed. Further, we should emphasize the need for primary and secondary prevention of fracture in patients using GC.
AS and SX created the study design. HIR and JJ searched and screened the titles in databases. AS and MMEA drafted the article. All authors read and approved the final draft.
None.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.