To determine the prevalence of diabetes mellitus (DM) in patients with essential hypertension attending outpatient medical clinic in a rural community setting of a developing country.
This cross-sectional study included patients of a medical outpatient clinic in Federal Teaching Hospital Ido Ekiti, Ekiti State, Nigeria. Study patients were consecutively recruited from Cardiac clinic and baseline investigations obtained from the patients' files were evaluated including Fasting Blood Glucose (FBG).The estimated minimum sample size was 80 with probability of type 1 error, assumption of α = 0.05 and power of 95%. A total of 160 patients with the minimal number of investigation results were included.
The 160 patients (76 male and 84 female) had a mean age (± standard deviation) of 64.6 ± 12.4 years (range, 40-88 years). The majority of the participants (50%) were aged 60-79 years. Diabetes was present in 25.7% (p < 0.001) of cases of hypertension with FBG test. Most of the patients (89.5%) were of low income earnings.
Our findings showed a high prevalence of DM among hypertensive outpatients in rural and low income setting.
Diabetes, Hypertension, Prevalence, Rural, Outpatient
Hypertension and diabetes mellitus (DM) are prevailing at an epidemic proportion throughout the world [1-3]. This is partly due to continuous change in lifestyle that favours the development of the conditions [4-7]. This co-morbidity has markedly increased the risk of cardiovascular disease (CVD) [8,9].
Diabetes is the commonest co-morbidity of hypertension. These diseases exert huge burden of demands on individuals, families, communities and the health system of any country. Research had shown that people with elevated blood pressure are at increased risk of diabetes [10].
Cardiovascular disease is at least two times more frequent in hypertensive patients with DM than in those with hypertension alone [11-15]. Early detection and treatment of DM in hypertensive patients may be particularly important to reduce the risk of developing cardiovascular complications, which may further lead to increase in morbidity and mortality rates [1].
The prevalence of hypertension depends upon the definition of hypertension. The prevalence data presented in this paper should be interpreted according to the definition of hypertension at the time the study was done unless otherwise stated.
An estimated 1.13 billion people worldwide have hypertension, most (two-thirds) living in low- and middle-income countries [2]. In Nigeria hypertension is estimated to be 28.9% [16].
Studies in Nigeria have reported that the prevalence of diabetes varies across different zones of the country but ranges from 2.2-9.8% [17-20]. The diabetes statistics of the International Diabetic Federation (IDF) showed that Nigeria has the highest number of people living with diabetes and impaired fasting glucose (IFG) in Africa [21].
However, there are scarce data on prevalence of diabetes in patients with hypertension in Nigeria. To the best of the authors' knowledge, no available data for rural tertiary hospital. Few data available are studies on prevalence of the co-morbidity especially from urban tertiary health centres [22,23]. Therefore, we sought to determine the prevalence of diabetes in adult hypertensives attending Medical Outpatient Clinic in a tertiary hospital located in a rural setting.
This study was a cross-sectional study on hypertensive outpatients with no identifiable secondary cause in a Federal Teaching Hospital Ido Ekiti, Ekiti State, Nigeria; located in a rural community of Ekiti state in Nigeria. We used non-randomised convenience sampling technique. Eligible patients were consecutively recruited in the study.
The inclusion criteria for enrolment include all patients that had undergone hypertension treatment attending the clinic during the period of study and aged more than 20 years. Patients with incomplete data were excluded and 160 patients were included in the final analysis.
Information were obtained from the patients' files by the senior resident physicians between year 2017-2018 which included demographic characteristics, blood pressure (BP), fasting blood glucose, smoking status, duration of hypertension, history of DM, anthropometric measurement and other relevant information.
Documented data in the case file were obtained in our clinic by standardized method. Weights (kg) were measured by standardized techniques and equipment [24]. Blood pressure was measured with a mercury sphygmomanometer at least twice in each participant with at least five minutes of rest in between, with the subject seated in a chair and relaxed, the back supported, and the arm at heart level. During the initial screening, BP of both arms was measured with mercury sphygmomanometer and the arm with higher BP used subsequently. Fasting blood glucose and fasting lipid profile were determined using venous blood by conventional laboratory devices after an overnight fast.
The following definitions were adopted for this study. Hypertension: Persistently elevated blood pressure (BP) ≥ 140/90 mmHg, based on at least two readings on separate occasions after the initial screening [25]. The choice of 140/90 mmHg as a cut-off point is based on the Seventh Report of the Joint National Committee on prevention, detection, evaluation and treatment of high blood pressure (JNC 7) criteria [25]. Diabetes mellitus: Diagnosed according to the World Health Organization diagnostic criteria [26].
Dyslipidaemia: National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III) cut-off points were used to identify participants with desirable, borderline high and high levels of lipoprotein risk factors [27]. Smoking and alcohol: Considered present if reported up to the day of the interview. Metabolic syndrome was defined according to WHO definition [28].
There is no national data for prevalence of DM among hypertensive patients. Therefore, we estimated the sample size based on average prevalence of 6% for DM for the Nigerian general population [17-20]. The estimated minimum sample size was 80 with probability of type 1 error, assumption of α = 0.05 and power of 95%.
Categorical variables were represented as number (percentage) and were compared by χ2 test. Continuous variables were represented as the mean (SD) in case of normal distribution, and were otherwise presented as medians. A p value less than 0.05 was considered as statistically significant. Ethics and research committee approval from the institution was obtained. Data were managed and analysed using SPSS for windows version 16 (SPSS Inc. Chicago, Illinois, USA).
A total of 160 patients entered the final analysis. Only 136 could afford to do FBG; 86 did TC and HDL; and 84 did LDL. There were 76 males (47.5%) and 84 females (52.5%) with an average age of 64.6 ± 12.4 years. Majority of the patients were low income earners (60.7%): petty traders, 23.8%; retirees, 22.5%; peasant farmers, 14.4%. Table 1 provided further summary of the baseline characteristics.
Table 1: Baseline characteristics of the study hypertensive outpatients. View Table 1
In hypertensive outpatients in this study, the prevalence of DM was 25.7%, higher in males (15.4%) than females (10.3%) as shown in Table 2. The table further shows that the prevalence of DM was high in patients with metabolic syndrome (61.5%) and increased with duration of hypertension from 24.1% in those of less than 5 years duration to 32% in those of equal or greater than 10 years. This trend was significant, p value less than 0.005.
Table 2: Detection rates of diabetes in hypertensive outpatients. View Table 2
Figure 1 shows a progressive increase in prevalence of hypertension and hypertensive diabetic with age. In both clinical conditions, there was a fall in prevalence in females of more than 65 years compared to 45-65 years of age.
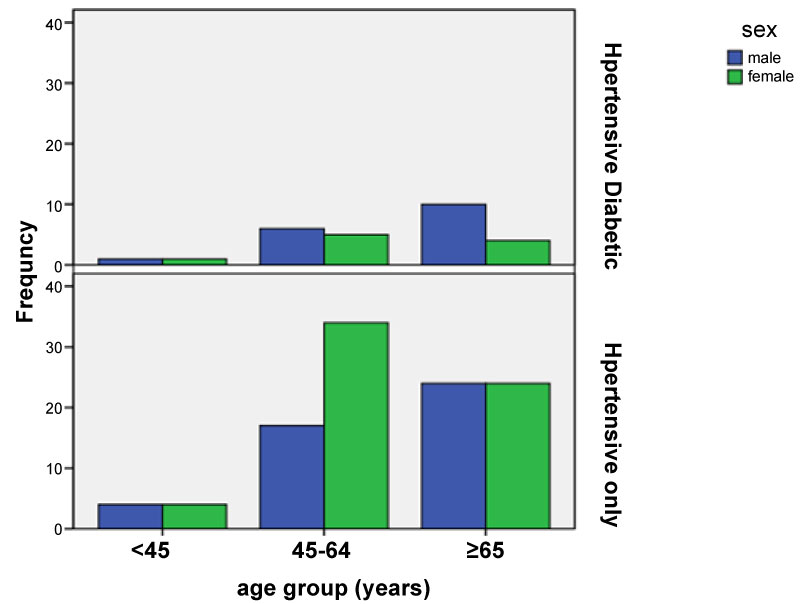 Figure 1: Sex and age distribution of hypertensive patients with and without diabetes mellitus.
View Figure 1
Figure 1: Sex and age distribution of hypertensive patients with and without diabetes mellitus.
View Figure 1
This study revealed a high prevalence of DM among medical outpatients with hypertension (25.7%) in a tertiary hospital situated in a rural community. This finding is similar to that reported in other studies done in urban centres [29-31].
However, the prevalence of DM in this study (25.7%) among hypertensive patients receiving treatment is much higher than newly diagnosed DM (2.2-9.8%) without hypertension in the study done across different zones of the country [17-20]. It is worthy of note that in this study, the longer the duration of hypertension the higher the prevalence of diabetes. These further established the fact that hypertension is a factor in the development of DM [14,32]. In addition, these evidences suggest that the strengthening of DM screening among patients with hypertension is crucial beyond initial screening for DM at diagnosis of hypertension.
Nevertheless, the study population is predominantly elderly as expected in age distribution for hypertension and diabetes [33-35]. In addition, rural populations are predominantly old population because of rural to urban migration [36]. The middle aged showed higher female gender prevalence of hypertension alone in this study compared to the elderly contrary to the established gender distribution [33]. This may not be unconnected with some published observations in South-West Nigeria and other parts of the world that females have better health seeking behaviour than males [37,38]. It has also been anecdotally found that mothers tend to get more attention from their children than the fathers hence, better health care.
The patients were predominantly low income earners. This is a triple tragedy of poverty, diabetes, and hypertension for these individuals to cope with. More so, the low income earning might have affected their treatment compliance in terms of affordability. This might have partly resulted into the observed high mean value of BP and sub-optimal mean value of FBG despite treatment. The outcome of such situation will be high morbidity and mortality in a rural setting of this nature.
There was a progressive increase in prevalence of hypertensive diabetic or hypertension alone with age as previously reported [33-35]. This was only observed in male unlike in female with a fall in prevalence for both clinical conditions in the elderly compared to the middle aged. The aforementioned health seeking behaviour of females and the generous care commonly received by aged mothers from their children unlike the aged fathers noted in this part of the world may partly be responsible for this observation.
However, there was no difference in gender distribution for young adults in both hypertensive-diabetic and hypertension alone. Similarly, there was no gender difference in the elderly for hypertension alone. These results agree with recent data [39,40].
Epidemiologic studies provide evidence for co-existence of hypertension and diabetes and possibly point towards a common genetic and environmental factor promoting both diabetes and hypertension [41]. Similarly, clustering of hypertension, insulin resistance or type 2 diabetes, hyperlipidaemia and central obesity have been documented in several populations [41]. Insulin resistance, increased tissue inflammation and reactive oxygen species (ROS) production resulting in endothelial dysfunction, increased tissue renin- angiotensin-aldosterone system (RAAS) and increased sympathetic nervous system activity have all been implicated in this complex pathophysiology of diabetes and hypertension.
It is estimated that about 25-47% of persons with hypertension have insulin resistance or impaired glucose tolerance [42]. With insulin resistance, there are impaired biological and physiological tissue responses to insulin. The relationship of insulin resistance, diabetes and hypertension is complex and interrelated. Untreated patients with essential hypertension have higher fasting and postprandial insulin levels than age- and sex-matched normotensive persons, regardless of body mass; a direct correlation between plasma insulin levels and BP exists [43,44]. Interestingly, the relationship between hyperinsulinaemia and hypertension is not seen in secondary hypertension [44]. This indicates that insulin resistance and hyperinsulinaemia are not consequences of hypertension, but rather a genetic predisposition that acts as a fertile soil for both diseases. This notion is supported by the observation that there is abnormal glucose metabolism in the offspring of hypertensive parents [44,45]. Thus, there is a strong association between hypertension, diabetes and insulin resistance. There is also a strong association between upregulation of RAAS, hypertension and diabetes [46-48]. This upregulation of RAAS results in enhanced generation of ROS and may explain impaired glucose utilisation as well as hypertension associated with insulin resistance and type 2 diabetes [49].
The potential limitations of this study require consideration. First, our sample consists of those referred to specialist clinic therefore may not reflect the status of those in the general population.
Second, two hours postprandial glycemic values were not considered in this study; hence, the actual prevalence might be underestimated. Third, the exact onset of DM could not be ascertained in some of the patients; hence, this favors a potential overestimation. Fourth, no consideration given to patients with impaired fasting glucose for definitive diagnosis with the use of oral glucose tolerance test, which may underestimate or overestimate the prevalence of DM.
In conclusion, our findings demonstrate that 25.7% of hypertensive outpatients had concomitant DM and the longer the duration of hypertension the higher the prevalence. Virtually all the patients were of low income status. This finding in a rural setting demands urgent deployment of health insurance scheme to ensure access to optimal health care. This will reduce potential morbidity and mortality.