Worldwide, the prevalence of vitamin D deficiency is as high as almost 50% among the elderly and the association between vitamin D and heart failure is widely debated.
To study the relation between serum 25-hydroxy vitamin D levels and echocardiographic parameters of cardiac function in heart failure patients with cardio-renal syndrome.
The study included 90 patients of all age groups and both sexes. They were divided into 3 groups of patients: 1: Systolic dysfunction and renal insufficiency (30 patients), 2: Systolic dysfunction only (30 patients). 3: Renal insufficiency only (30 patients). The Patients were subjected to full comprehensive echocardiography and kidney function tests with estimation of creatinine clearance, and measurement of Vitamin D level that was statistically studied against echocardiographic parameters of cardiac systolic and diastolic function.
Vitamin D deficiency (< 20 ng/ml) was significantly higher in patients with cardio-renal syndrome. Patients with vitamin D deficiency had a significantly higher left ventricular thickness and higher left ventricular (LV) mass which seems to be linked eventually to worse outcomes. The Worsening Diastolic function is still a matter of conflict but, at least in our study, Vitamin D deficiency was not associated with worse left ventricular diastolic dysfunction or abnormal left atrial volume (LAV) index. Due to highly significant negative correlation between mean wall thickness and Vitamin D level, a Roc curve was done revealing a sensitivity of 80% and a specificity of 72% for the mean wall thickness (≥ 10 mm) to identify patient with vitamin D deficiency.
Vitamin D deficiency is highly prevalent in heart failure patients (especially those with cardiorenal syndromes) and has been shown to be associated with increased cardiovascular diseases, including left ventricular hypertrophy (higher LV thickness and mass) and heart failure.
Cardiorenal syndrome, Vitamin D, Heart failure
BNP: Beta Natriuretic Peptide; LV: Left Ventricle; IVS: Interventricular Septum; LVH: Left Ventricular Hypertrophy; LAV: Left Atrial Volume; EDV: End-Diastolic Volume; ESV: End-Systolic Volume; EDD: End-Diastolic Diameter; ESD: End-Systolic Diameter; Cr Cl: Creatinine Clearance; Na: Sodium; K: Potassium; IVRT: Isovolumetric Relaxation Time; EF: Ejection Fraction; PW: Posterior Wall
Worldwide, the prevalence of vitamin D deficiency is as high as almost 50% among the elderly. A recent meta-analysis showing that vitamin D supplementation significantly decreased all-cause mortality raised the public health interest in vitamin D. The classic role of vitamin D for maintaining bone health was recently extended by reports linking vitamin D deficiency to various other diseases, including arterial hypertension and diabetes mellitus [1]. Cardiomyocytes express the vitamin D receptor, and studies in rodents have shown that vitamin D protects against cardiac hypertrophy and myocardial dysfunction [2].
Although many studies have reported that renal insufficiency is associated with adverse cardiovascular outcomes, renal dysfunction is still an underappreciated prognostic factor in heart failure and renal insufficiency is commonly viewed as a relative contraindication to some proven efficacious therapies [3]. The mechanisms by which decreased renal function is correlated with cardiovascular disease are unknown. The cardiorenal syndrome is divided by Ronco, et al. into five subtypes: Type 1 describes acute decompensated heart failure leads to acute kidney injury. Type 2 is characterized by chronic heart failure that leads to chronic kidney disease. Type 3 describes acute kidney injury that leads to acute cardiac dysfunction or heart failure. Type 4 is characterized by primary chronic kidney disease that contributes to cardiac dysfunction. Type 5 is combined heart and kidney dysfunction due to systemic disorders such as sepsis [4].
The association between vitamin D and cardiovascular disease events is widely debated and analyzed in the recent literature. In a very recent cross-sectional study, Pilz, et al. measured 25(OH)D [25(OH)Vitamin D] levels in 3,299 Caucasian patients who were routinely referred for coronary angiography. They found that vitamin D is associated with prevalent myocardial dysfunction, heart failure, and sudden cardiac death [5]. Although the link between vitamin D deficiency and cardiovascular disease may be, in part, mediated through elevated PTH and calcium-phosphate metabolism, recent scientific evidence showed that vitamin D has 3 major potential protective mechanisms First, experimentally, it could directly suppress renin gene expression [6]. Second, the presence of vitamin D receptors in the cardiac muscle cells, a calcitriol-dependent Calcium binding protein and a calcitriol-mediated rapid activation of voltage-dependent calcium channels [7,8]. Third, vitamin D deficiency triggers secondary hyperparathyroidism, which then directly promotes cardiac hypertrophy [9].
To study the relation between serum 25-hydroxy vitamin D levels and echocardiographic parameters of cardiac systolic and diastolic function in heart failure patients with and without cardio renal syndrome.
The study included 90 patients of all age groups and both sexes, admitted to Ain-Shams University hospital. The patients were classified to 3 groups:
Group 1 included 30 patients with LV Systolic dysfunction and renal insufficiency, Group 2 included 30 patients with LV Systolic dysfunction only and Group 3 included 30 patients with renal insufficiency only. Inclusion criteria was those with systolic heart failure defined as having symptoms and signs typical of heart failure in addition to reduced LVEF (less than 40%), and with renal impairment defined as having creatinine clearance less than 60 mL/kg/min. While the following patients were excluded: Patients with previous vitamins D supplementations, those with conditions known to cause Vitamin D deficiency e.g., malignancies, chronic liver disease, bowel disease causing mal-absorption, patients with hemodialysis, etc., patients with marked poor echogenicity and patients on hemodialysis.
After oral consent from the patients, all patients were subjected to thorough history taking including onset of symptoms, etiology of heart failure, NYHA class on presentation to the hospital and risk factors. Full clinical examination was done for signs of heart failure and sings of renal failure. Pelvi-abdominal ultrasound was done for grading of nephropathy: Grade 0: Normal renal echogenicity with maintained corticomedullary definition. Grade 1: Echogenicity is the same as that of the liver with maintained corticomedullary definition. Grade 2: Echogenicity greater than that of the liver with maintained corticomedullary definition. Grade 3: Echogenicity greater than as that of the liver with poorly maintained corticomedullary definition. Grade 4: Echogenicity greater than as that of the liver with loss of corticomedullary definition [10].
Venous sampling Samples were sent for laboratory measurements of BNP, 25(OH) vitamin D (using ELIZA technique) (deficiency defined as serum levels less than 20 ng/ml) [11], Calcium, and Phosphorus, Sodium, Potassium and serum creatinine. Estimated Glomerular filtration rate was calculated using the Cockcroft-Gault equation, which has been validated in patients with a wide variety of medical diagnoses, and patients with Renal impairment will be classified into 5 groups as described below [12]: Grade 1: GFR above 90 ml/min. It usually describes a Normal kidney function but urine findings or structural abnormalities or genetic trait point to kidney disease. Grade 2: GFR of 60-89 ml/min. It usually describes mildly reduced kidney function, and other findings (as for stage 1) point to kidney disease. Grade 3: GFR of 30-59 ml/min. It usually describes a moderately reduced kidney function. Grade 4: GFR of 15-29 ml/min. It usually describes a severely reduced kidney function. Grade 5: GFR less than 15 ml/min. It usually describes a very severe, or end stage kidney failure.
Trans-thoracic Echocardiography was done for all patients using available echocardiography systems equipped with a 2.5 multi-frequency phased array transducer (Vivid 5 or 7, GE-Vingmed, Horton, Norway). Digital routine grayscale 2-dimensional apnea from tissue Doppler cineloops from 3 consecutive beats will be obtained at end expiratory apnea from standard apical views at depth of 12-20 cm. gain settings will be adjusted for routine grayscale 2D and tissue Doppler cineloops will be obtained ,including mid-LV short axis views at the level of papillary muscle and standard apical views (4-chamber, 2-chamber, and long-axis) sector width was optimized to allow for complete myocardial visualization while maximizing the frame rate. LV end-diastolic volume (EDV), LV end-systolic volume (ESV) and ejection fraction (EF%) were obtained with the modified biplane Simpsons method from apical 2- and 4-chamber images. Left atrial volume was calculated using the prolate ellipse method [13]. All measurements will be made in ≥ 3 consecutive cardiac cycles and in ≥ 5 cycles if the patient rhythm was AF and average values will be used for the final analysis. The pulsed wave Doppler-derived trans-mitral flow profile, pulmonary venous flow profile, and digital color tissue Doppler-derived mitral annular velocities will be obtained from the apical 4-chamber view. The ratio of mitral flow early diastolic wave (E) to late diastolic atrial contraction wave velocity (A), or E/A ratio; and the E wave deceleration time (E-DcT) will be measured. In addition, the ratio of early diastolic mitral flow velocity to TDI-derived early diastolic mitral annular velocity (E/e') ratio will be calculated. LV systolic function will be assessed by EF%, ESV, and TDI derided peak systolic velocity (s'). LV diastolic function will be assessed using E/A ratio, E-DcT, E/e', left atrial volume (LAV), and pulmonary venous indices.
The study was accepted by the Ethical Committee of Faculty of Medicine, Ain Shams University.
Categorical data were expressed as number (%). Continuous data were expressed as mean ± SD and were compared using Student t-test. Correlations were checked for echocardiographic parameters against vitamin D level using Person correlation coefficient. With the exclusion of parameters that show co-linearity, multivariate regression analysis was done to check for the best independent predictor of vitamin D level. The ability of different echocardiographic parameters to detect vitamin D level < 20 ng/ml was done using receiver operator characteristics curve (ROC-curve), by which the best cutoff value for prediction that shows best sensitivity and specificity was checked. Binominal logistic regression was done to check the hazard ratios in case of Vitamin D < 20 ng/ml. P-value < 0.05 was considered statistically significant. All the analyses were performed with commercially available software (SPSS version 21.0, SPSS, Inc., Chicago, IL, USA).
Table 1 shows that the mean age for patients was 57.2 ± 8.1 years. 23 of them (25.6%) were females. All patients were in sinus rhythm, 35 patients (38.9%) were diabetics, 41 patients (45.6%) were hypertensive, 55 patients (61.1%) were smokers, and 13 patients (14.4%) were hyperlipidemic.
Table 1: Comparing the baseline characteristics between patients' subgroups. View Table 1
31 (34.4%) Patients were admitted with a NYHA class 1, 42 (46.7%) patients were in NYHA class II, 15 patients were in NYHA class III (16.7%) and 2 patients (2.2%) were in NYHA class IV. There was no significant difference regarding age (P value 0.055) or presence of Hypertension (P value 0.8), or smoking (P value 0.54). But there were higher Diabetic patients in group 3 (17 patients) compared to 1 (15 patients) or group 2 (only 3 patients), (P value 0.01), which reflects the effects of DM on worsening KFT among the study population.
As shown in Table 2, Compared to group 2 (HF only patients) and group 3 (RF only patients), group 1 (HF + RF patients) had a significant lower Vitamin D level (mean 14.34 ± vs. 22.6 ± 8), lower Sodium (mean 123.7 ± 5 vs. 126.8 ± 3) and Calcium (mean 8.01 ± 0.33 vs. 8.42 ± 0.32) levels and higher Phosphorus (mean 7.67 ± 2.2 vs. 4.02 ± 1.19) levels. No significant differences (P-value 0.89) regarding BNP level between groups 1 and 2 while there was no significant differences (P-value 0.89) regarding creatinine and Potassium levels (P-value 0.1 and 0.56 respectively) between groups 1 and 3. A significant difference in Vitamin D deficiency was noticed being higher in group 1 (96.7%) than group 3 (76.7%) and lesser incidence of deficiency in group 2 (43.3%) Table 3 and Figure 1.
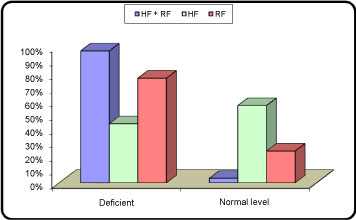 Figure 1: Comparisons between patients' subgroups regarding vitamin D deficiency.
View Figure 1
Figure 1: Comparisons between patients' subgroups regarding vitamin D deficiency.
View Figure 1
Table 2: Comparisons between patients' subgroups regarding laboratory results. View Table 2
Table 3: Comparisons between patients' subgroups regarding vitamin D deficiency (< 20 ng/ml). View Table 3
1. Regarding Diastolic function: It was found a positive correlation between vitamin D with IVRT in all patients group (r = 0.308, p = < 0.003) and similarly in group 1 (r = 0.363, p < 0.049) and group 2 (r = 0.670, p < 0.001). Contradicting this finding was the negative correlation between Vitamin D level and e' (r = -0.292, p < 0.005) in all patients group and in group 1 ( r = -0.659, p < 0.001) and E/e .but owing to lack of significant correlations between vitamin D and other diastolic parameters as LAV, average a', E wave velocity, A wave velocity, E/A ratio, E-DcT and e'/a', it is suggested that there are no consistent correlation between vitamin D and diastolic dysfunction.
2. Regarding systolic function: Vitamin D level correlated negatively with average s' velocity (r = -0.285, p < 0.007), this was confirmed in group 3 patients (r = -0.667, p < 0.001). There was a positive correlation with ESD and ESV in group 3, but could be explained as these patients tend to have higher LV internal dimensions by the effect of hypervolemia the often coexist in Renal failure patients no significant correlations with other systolic parameters.
3. Regarding LV wall thickness: It was found that there was a significant negative correlation between vitamin D and all parameters of LV wall thickness. In all patients' group a Significant negative correlation was also noticed for IVS thickness (r = -0.567, p < 0.001), PW thickness (r = -0.691, p < 0.001), mean wall thickness (r = -0.654, p < 0.001), and LV mass (r = -0.304, p = 0.004), this was evidenced in group 1 for IVS thickness (r = -0.514, p = 0.004,), PW thickness (r = -0.800, p < 0.001), mean wall thickness (r = -0.714, p < 0.001), and LV mass (r = -0.609, p < 0.001). And similarly in group 2 for PW thickness (r = -0.480, p = 0.007), mean wall thickness (r = -0.385, p = 0.03), with a non-significant trend toward lower IVS wall thickness and LV mass and for group 3 for IVS thickness (r = -0.833, p < 0.001), PW thickness (r = -0914, p < 0.001), mean wall thickness (r = -0.898, p < 0.001), and LV mass (r = -0.594, p < 0.001).
As expected from vitamin D metabolism, it has a highly significant positive correlation with creatinine clearance and Calcium level there was a negative correlation with Phosphorus level that could be explained by impaired Phosphorus excretion in advanced CKD. It is worth noting that BNP level did not correlate with vitamin D levels overall denoting a neutral action on the neurohormonal disturbance accompanying HF.
Table 4: Correlations between laboratory results and echocardiographic determinants of systolic, diastolic function and LV wall thickness versus vitamin D levels. View Table 4
In all patients' groups, there was a significant negative correlation between creatinine clearance and IVS thickness, PW thickness, Mean wall thickness, and S” (with more impaired KFT, the more LVH and increase contractility), there was a significant positive correlation between creatinine clearance and E/A ratio (denoting worsening diastolic function with worsening KFT), (P value 0.037), observed also a positive correlation with EDD, ESD, EDV, EDV in group 3 patients indicating that patients with CKD had dilated LV internal dimensions and volumes.
Table 5: Correlation between creatinine clearance and echocardiographic measurements. View Table 5
By applying a multivariate linear regression model for predictors of vitamin D level among patients, it was found that IVS, PW and mean LV wall thickness were independent predictors of vitamin D deficiency, (beta = 0.546, 0.622, 0.629, p = 0.001, < 0.001, < 0.001, respectively) (Table 6).
Table 6: Echocardiographic predictors of vitamin D deficiency. View Table 6
Next, ROC-curve was initiated to check the ability of these variables to detect vitamin D less than 20 ng/ml and it was found that mean wall thickness could do so with the best area under the curve (AUC) (AUC: 0.826, cut-off values: ≥ 10 mm, sensitivity: 80%, specificity: 72.3% respectively) (Figure 2).
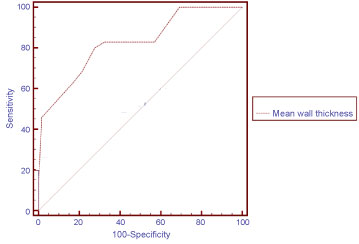 Figure 2: ROC curve for predictors of Vitamin D < 20 mcg/ml.
View Figure 2
Figure 2: ROC curve for predictors of Vitamin D < 20 mcg/ml.
View Figure 2
Our study demonstrated that the prevalence of vitamin D deficiency (defined as a 25-OHD level less than 20 ng/ml) [11] is increased among patients with heart failure than in general population and is much increased in patient with combined Heart and renal failure compared to either Heart failure or renal failure alone.
The increased prevalence of vitamin D deficiency in heart failure is multifactorial. Heart failure itself can induce vitamin D deficiency by several mechanisms. Shane, et al. had demonstrated decreased cutaneous photo-conversion of vitamin D in patients with severe heart failure who have difficulty ambulating and obtaining adequate sunlight exposure [14]. Right ventricular failure cause intestinal edema leading to decreased Vitamin D absorption together with passive liver congestion that impairs the synthesis of 25-OHD, making less substrate available for downstream activated vitamin D production in the kidney [15]. Finally Renal Insufficiency, that is prevalent in patients with Heart failure, decreased the enzymatic conversion of 25-OHD to the active form 1,25(OH)2D [16].
In our study vitamin D was associated with increased LV wall thickness and LV mass, with a little effect on cardiac systolic function (at least early in the diseases).
Several studies had shown that 25(OH)D deficiency is a frequent finding in essential hypertension patients and is independently associated with LVH . In a study by Fallo, et al. on 62 newly diagnosed hypertensive, compared with 24 healthy normotensive sex-, age- and BMI-matched controls. Hypertensive patients with 25(OH) D deficiency, had higher prevalence of LVH than their 25(OH) D-sufficient counterparts with no differences between the two groups were found in blood pressure levels as well as in other biochemical and hormonal parameters. There was an inverse correlation between LV mass index and 25(OH) D levels [17].
Further, in patients with coronary calcifications, lower vitamin D levels were found to be associated with higher LV relative wall thickness [18]. Same findings were also supported by another study of patients on hemodialysis [19].
The association of vitamin D deficiency with LVH had several possible mechanisms. Increased PTH levels are associated with insulin resistance, inflammation, vascular stiffness, myocardial hypertrophy and fibrosis [20]. Moreover, through activation of the 1-25-OH vitamin D nuclear receptor, vitamin D has been shown to suppress myocardial hypertrophy and abrogate the hypercontractility of the myocardium that is observed with diastolic HF, this effect is attenuated in Vitamin D deficient patients [21,22].
Vitamin D deficiency predisposes to up-regulation of the RAS with subsequent hypertrophy of both the left ventricle and vascular smooth muscle cells [15]. In vitamin D deficient animals, there is increased incidence of Hypertension, LVH and atherosclerosis [9]. Human studies indicate that vitamin D inhibits renin synthesis, which leads to lower blood pressure [6].
Whereas the association between vitamin D and LVH was proven, our study found no consistent correlation between vitamin D and Diastolic function. The studies investigating the effects of vitamin D on LV diastolic functions are controversial and mostly are not suggestive of possible relation [23].
Vitamin D therapy has been reported to be associated with the reduction of LAV index and attenuation of a rise of BNP in patients with chronic kidney disease with mild-to-moderate LV hypertrophy [24].
On contrary, a study of 648 elderly individuals where serum levels of 25-OH D were reported as not being significantly associated with measures of LV geometry or function [25]. Another study not supporting a causal relationship of vitamin D to heart function and geometry was a double-blinded placebo controlled 48 week therapy clinical trial with Vitamin D in 227 patients with chronic kidney disease. The therapy did not alter LV mass index or improve measures of diastolic dysfunction [26].
The Hoorn study showed, in a prospective manner, that Vitamin D levels at baseline were not associated with decreased LAVI over a subsequent 8 years of follow-up. In addition, the lowest Vitamin D quartile had a significantly higher LVMI than the highest quartile. These results were attenuated by multivariable analysis. Of note, the Hoorn study did not measure other diastolic parameters including tissue Doppler measurements [27].
Anil Pandit, et al. conducted the first, large cross-sectional study attempting to examine any relationship between baseline Vitamin D levels and comprehensive echocardiography to study LV diastolic function at Mayo Clinic. A retrospective analysis of 1011 patients from 2005 to 2010 who had an echocardiogram and a serum Vitamin D level performed 1 month later. Patients were divided according to Vitamin D deficient and vitamin D sufficient groups. With the exception of Diabetes, Patient demographics and conventional CVD risk factors of hypertension, smoking history and clinical coronary artery disease presence were comparable in both groups. The main finding of this study is that Vitamin D showed no significant correlation with diastolic function. Even in Diabetics who constitutes a higher proportion of patients. In the group of vitamin D deficiency, neither the Doppler diastolic parameters of LV diastolic dysfunction (which may subjects to acute hemodynamic changes) nor LAVI (which is a surrogate marker of long-term impaired diastolic dysfunction) were different between the study groups. Of note, the same study showed that there was a significant relationship between Vitamin D level and IVS and LV mass index after adjusting for age, hypertension and Vitamin D therapy status suggesting the role of Vitamin D level on ventricular remodeling [23].
There are several possible explanations for the lack of a relationship between Vitamin D levels and diastolic dysfunction. Most of the studies (including our study) were cross-sectional study. There was insufficient information to assess the duration of Vitamin D deficiency. To cause diastolic impairment, this deficiency may need to be of a much longer duration [28]. However, the Hoorn study examining serum Vitamin D level and LAVI after 8 years of follow-up failed to demonstrate any association between Vitamin D deficiency and diastolic dysfunction.
Surprisingly, another study by Rahman, et al. showed that, beside the increased wall thickness and worse systolic parameters in vitamin D deficient patients, higher vitamin D levels were associated with worse LV diastolic function in form of lower average e' velocity, higher E/e' ratio, and longer IVRT, all of which are all linked to worse diastolic functions and high left ventricular filling pressures [29]. Similarly Drueke, et al. and Zittermann, et al. Suggested that a biphasic dose-response curve exists for vitamin D, with adverse effects associated with very high and very low vitamin D levels. Vitamin D deficiency has been observed to induce myocardial hypertrophy and extracellular matrix production and deposition in myocardial tissue. Negative effects associated with vitamin D excess include hyperphosphatasemia, hypercalcemia, increased matrix metalloproteinase (MMP) levels, medial calcification, and arterial stiffness [30,31].
Vitamin D deficiency is highly prevalent in heart failure patients (especially those with cardiorenal syndromes) and has been shown to be associated with increased cardiovascular diseases, including LVH and heart failure. Patients with vitamin D deficiency had significantly higher ventricular thickness (IVS, PW and mean wall thickness) and higher LV mass but it was not associated with worse LV diastolic function or abnormal LAV index (Table 7).
Table 7: Sensitivity and specificity of ROC to predict Vitamin D level < 20 ng/ml. View Table 7
Unfortunately, the study suffered the following limitations:
1. Small sample size with smaller subgroup numbers, further studies should include larger number of patients in each subgroup to check if the findings of our results can be reproduced.
2. A study of the molecular mechanisms (including PTH level) to elaborate the mechanisms noticed for vitamin D effects were not done, thus further studies should address this issue.
3. The study, being a cross sectional study is lacking follow up to the patients to monitor outcomes in patients after correction their vitamin D status.
4. A more specific systolic and diastolic parameters e.g. strain and strain rate, early LV filling flow propagation slope (Vp) could have helped in identifying the exact relation between vitamin D and cardiac systolic and diastolic function.