Introduction: Cystic VS is a particular anatomo-clinical entity. Tumor resection must consider the preservation of neurological function and the risk of hematoma of the operating cavity.
Material and methods: We present a retrospective study of 12 cystic vestibular schwannomas among 32 vestibular schwannomas operated between September 2019 and December 2021.
The tumor volume and the quality of the tumor resection were evaluated by MRI according to the Kenzaki classification. The facial nerve function was evaluated according to the House and Brackmann classification. The type of the cystic SV according to the position of the cyst and the thickness of the cystic wall was classified into type A and B according to the Piccirillo, et al. classification (PC).
Results: All patients were operated by retro sigmoid approach in a semi-sitting position. The rapid onset and frequency of clinical signs is related to the volume of the SV cyst. Some postoperative complications characterize cystic SV, in particular: Hematoma of the operating cavity in 4 patients secondary to the hemorrhagic character of the solid part, worsening of low nerve function in 2 patients explained in our opinion by the vasospasm of the PICA and the worsening of the function of the facial nerve in 4 patients with cystic VS type B related to the fragility of the cystic wall.
Conclusion: Particular caution should be given to patients with cystic VS type B because of the rapid onset of clinical signs and the frequency of postoperative morbidity.
Cerebellar pontine angle (CPA), Cystic vestibular schwannomas (CVS), Facial and low nerve function, Hematoma
Vestibular schwannoma is a rapidly growing tumor, responsible for signs of brain stem compression. Apparently easy to operate, the wall of the cyst strongly adheres to the vasculo-nervous structures of the cerebellopontine angle, which explains the high incidence of postoperative facial paralysis and hematoma of the operative cavity. Due to its characteristics, the wait and scan attitude is not recommended, and the ideal treatment is surgery.
In this article, we report our experience in the management of cystic vestibular schwannomas. Clinical presentations, surgical outcomes, extent of tumor resection, facial nerve function, and postoperative morbidity, are reported.
During the period from September 2011 to December 2021, 622 VS have been operated by Professor Lotfi Boublata. We present a retrospective study of patients with cystic VS treated surgically during the period from September 2019 to December 2021 at the Neurosurgery department of Benbadis Hospital University, in Constantine, in the east of Algeria.
Clinical presentations, duration of symptoms, MRI characteristics, tumors sizes, surgical outcomes including extent tumor resection, facial nerve function, trigeminocardiac reflex (TCR) and surgical morbidities have been studied.
Tumor sizes and extent of tumor resection were evaluated according to the Kanzaki classification (Table 1) [1]. The facial nerve function was graded according to the House-Brackmann scale [2]. In cases where tumor removal is not total, the tumor residue should be followed-up by regular interval MRI.
Table 1: Cystic and solid vestibular schwannomas sizes according to the Kanzaki classification: All cystic VS were large or giant. View Table 1
All patients were operated by a retrosigmoid approach in a semi-sitting position.
The study included 32 patients with solid VS (72.8%) and 12 patients with cystic VS (27.2%) operated during 27 months. It’s important to remember that in our article published in 2017, we operated 152 large and giant VS in 5 years. The low number, in this study, is explained by the Covid crisis.
The mean patient age was 42 years (range 26-70 years). There are 05 Males, and 07 Females patients. The mean duration of symptoms was 19 months (range from 6 to 48 months).
The most common symptoms were (Table 2): Deafness: 10 cases, trigeminal palsy: 10 cases, low nerves dysfunction: 08 cases, cerebellar dysfunction: 08 cases, and intracranial hypertension: 04 cases.
Table 2: Preoperative clinical characteristics in patients with cystic and solid VS: Symptom duration and frequency of low nerves dysfunction is statistically significant in patients with cystic VS. View Table 2
There were 04 cases of cystic VS with symptomatic hydrocephalus, and the patients required the insertion of a ventriculoperitoneal shunt, during the preoperative period.
All our patients were operated on by the retro sigmoid approach in a semi-sitting position. The asterion was a good anatomic landmark which corresponds to the transverse sinus-sigmoid sinus junction. In the absence of the asterion, the digastric point is a constant landmark which corresponds to the posterior edge of the sigmoid sinus. A retrosigmoid craniotomy was performed to expose the inferior margin of the transverse sinus, that corresponds to the tentorium, the posterior margin of the sigmoid sinus which corresponds to the petrous posterior face, and the floor of the posterior fossa that will allow a quick and easy access to the cerebellomedullary cistern.
After opening the dura 02 situations are possible:
- In the first situation the cystic wall of the VS is immediately visible and facilitates access to the CPA: 01 case (Figure 1).
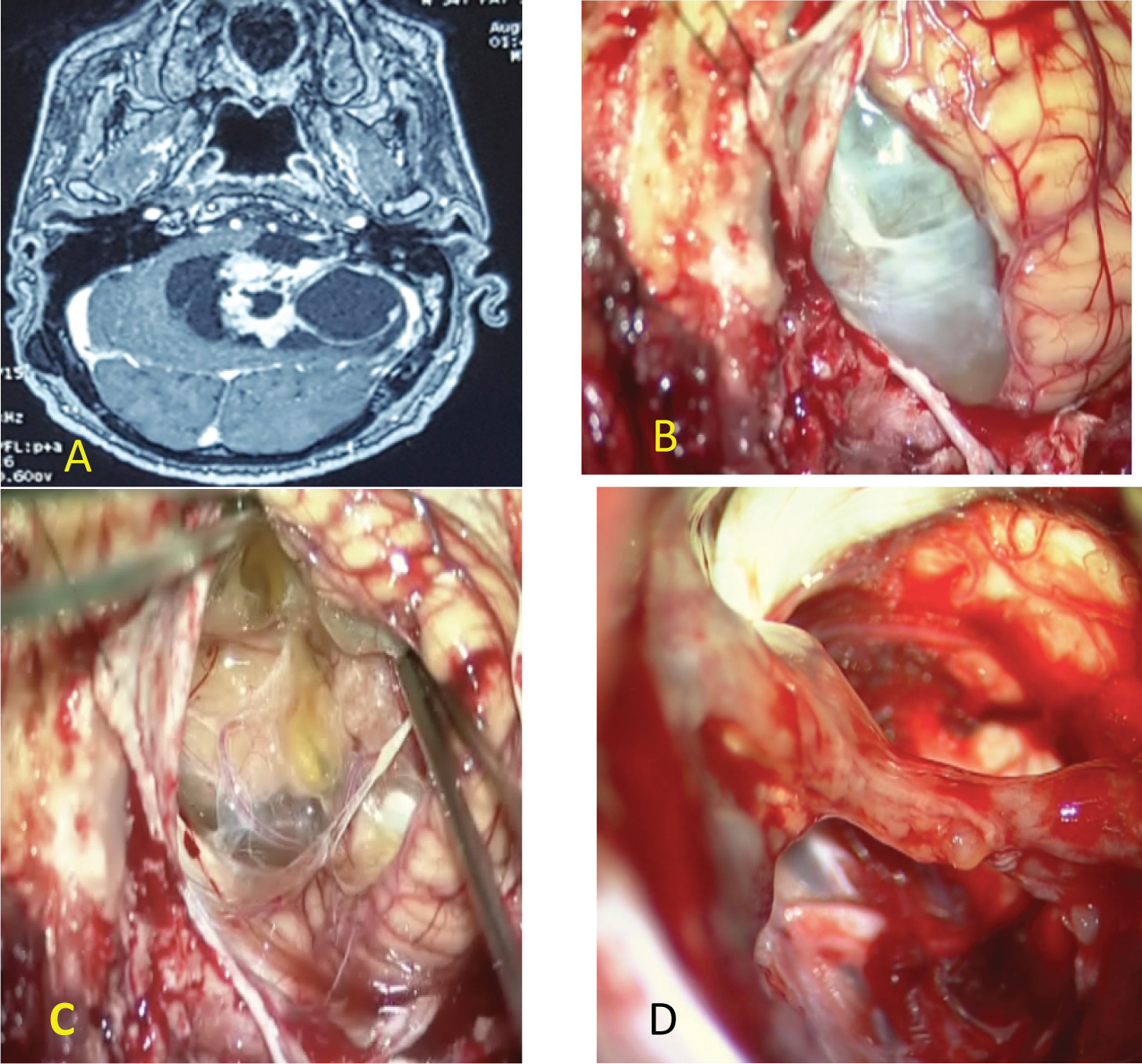 Figure 1: (A) MRI of giant cystic vestibular schwannoma; (B) The cystic wall of the VS is immediately visible; (C) Solid part of cystic VS; (D) Anatomic preservation of facial nerve and a thin layer of tumor was left over the facial nerve.
View Figure 1
Figure 1: (A) MRI of giant cystic vestibular schwannoma; (B) The cystic wall of the VS is immediately visible; (C) Solid part of cystic VS; (D) Anatomic preservation of facial nerve and a thin layer of tumor was left over the facial nerve.
View Figure 1
- In the second situation, to expose the tumor the cerebellum must be retracted with a narrow brain retractor and with a fine bayonet forceps the cerebellomedullary cistern was opened. The drainage of cerebro- spinal fluid (CSF) eased the cerebellum retraction: 11 cases (Figure 2).
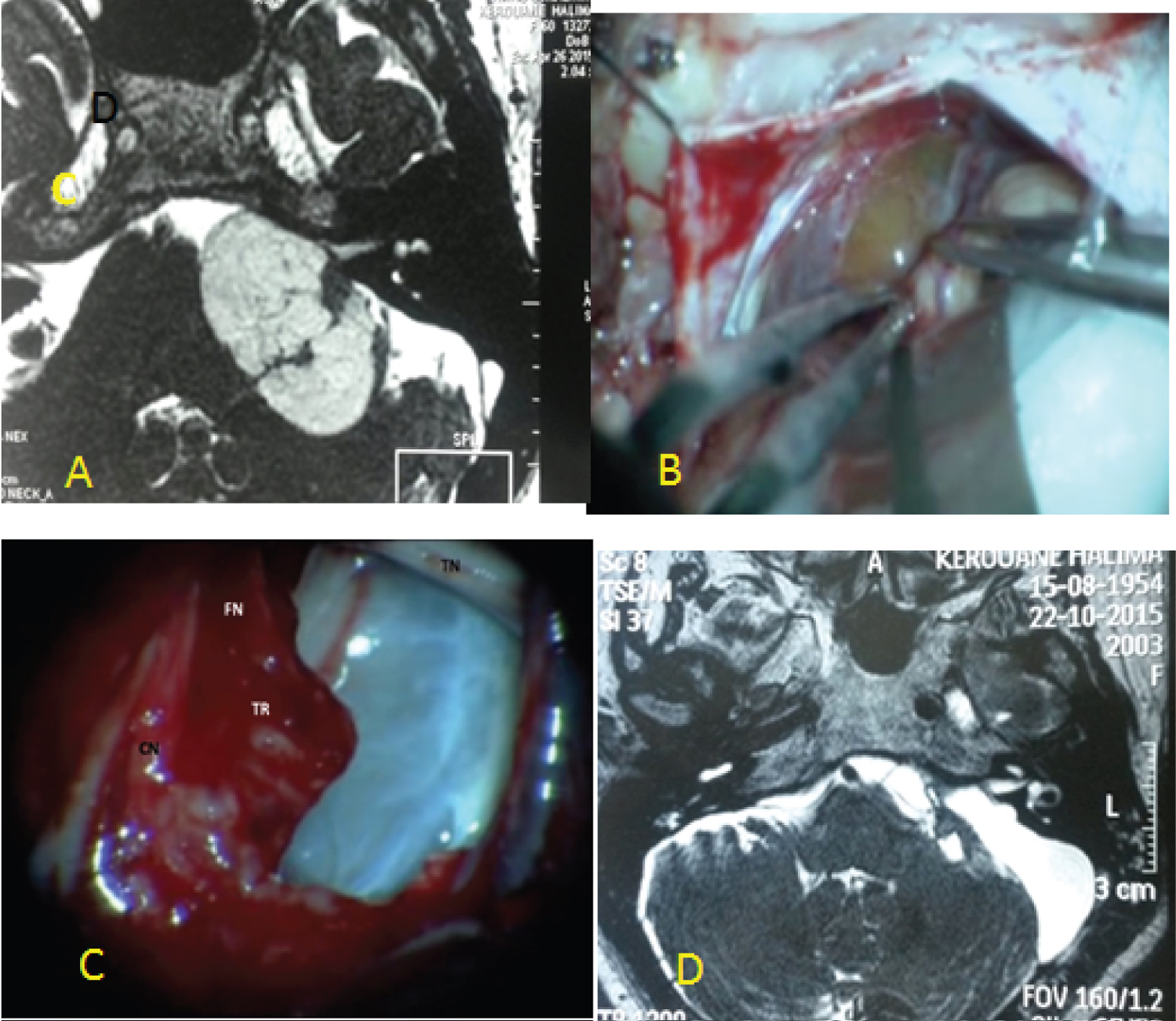 Figure 2: (A) MRI of giant cystic VS type B (PC); (B) Evidence of the cyst wall after cerebellum retraction; (C) anatomic preservation of facial and cochlear nerve and a thin layer of tumor was left over the facial nerve; (D) postoperative MRI showing near total resection of cystic VS.
View Figure 2
Figure 2: (A) MRI of giant cystic VS type B (PC); (B) Evidence of the cyst wall after cerebellum retraction; (C) anatomic preservation of facial and cochlear nerve and a thin layer of tumor was left over the facial nerve; (D) postoperative MRI showing near total resection of cystic VS.
View Figure 2
In cystic VS type A (PC) [3], identification and dissection of the facial nerve is less difficult in 03 cases (Figure 3). In type B (PC) the cyst is peripheral with a thin and fragile wall, making the establishment of a dissection plane more difficult (Figure 1 and Figure 2). When the facial nerve strongly adheres to the tumor, a thin layer of the tumor is left in place to preserve its integrity and especially its function 03 cases. In 2 cases the facial nerve was identified at the level of the brainstem but its identification on the wall of the cyst was impossible. For these 2 patients we proposed a facio-hypoglossal anastomosis.
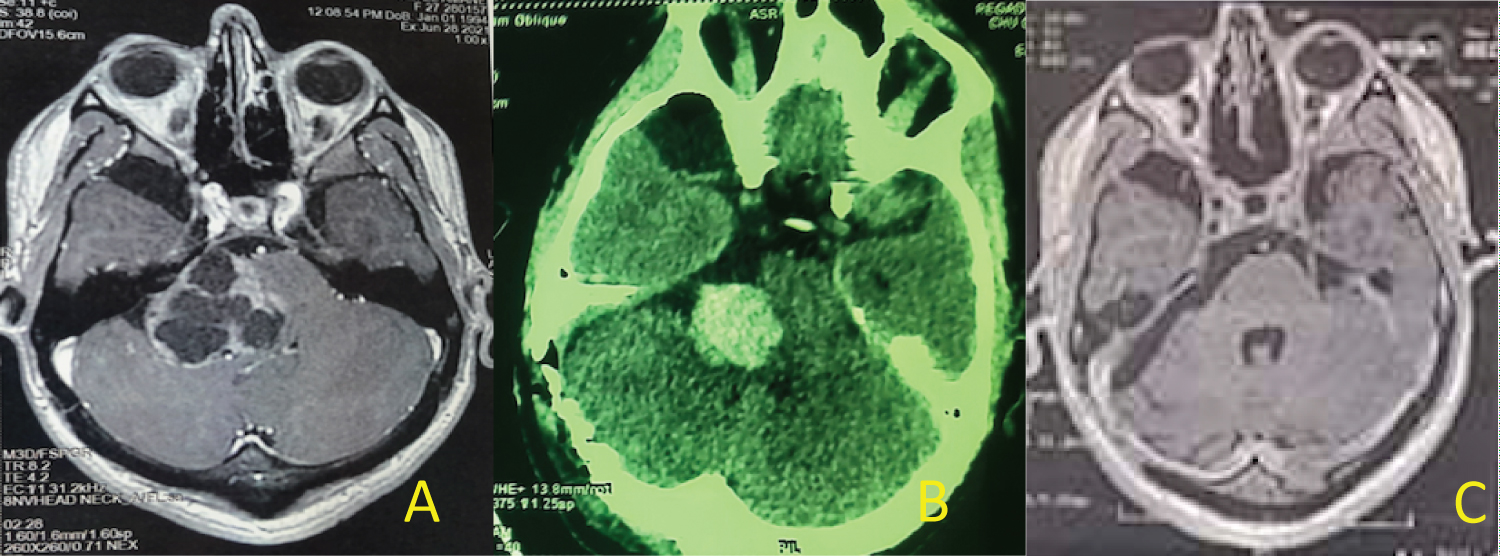 Figure 3: (A) MRI of giant cyst VS; (B) CT scan: Postoperative hematoma; (C) Postoperative MRI with great total tumor resection.
View Figure 3
Figure 3: (A) MRI of giant cyst VS; (B) CT scan: Postoperative hematoma; (C) Postoperative MRI with great total tumor resection.
View Figure 3
At the end of surgery meticulous hemostasis was performed and verified by jugular compression. In 02 cases the solid part of cystic VS bleeded and generally its removal ensures good hemostasis.
We adopt the philosophy of Prof. Samii: don’t use bipolar coagulation during the resection and the dissection of the tumor [4].
Postoperative morbidity was dominated by 4 major complications (Table 3):
Table 3: Postoperative morbidity in cystic and solid VS patients: The incidence of postoperative hematoma, hydrocephalus and low nerves dysfunction was statistically significant in patients with cystic VS. View Table 3
- Hematoma of operating cavity (04 cases): It was responsible in 02 patients for a delay in postoperative recovery with significant postoperative hematoma, accompanied in one patient by hydrocephalus. These patients required immediate evacuation of hematoma and ventriculoperitoneal shunt in case with hydrocephalus. The postoperative course was marked by a clinical improvement in the patient without hydrocephalus and the death of the patient with hydrocephalus. The 02 other patients were conscious without neurological disorders and were managed conservatively.
- Postoperative lower nerves dysfunction had worsened in 2 patients, and she required a tracheostomy.
- Facial nerve dysfunction (Table 4): In the immediate postoperative period, the function of the facial nerve had worsened in 3 patients (V, VI HB). In 02 cases the facial nerve was not identified and in 01 cases the facial nerve was preserved anatomically but the response to the stimulation was very weak at the end of the intervention.
Table 4: Facial nerve function in cystic and solid VS, at discharge and 6 months after surgery, according to the House Brackmann (HB) classification: The incidence of postoperative facial palsy was statistically significant in cystic SV. View Table 4
- Cerebrospinal fluid leak (04 cases): it is not a complication related to the nature of the lesion, but it is related to the approach. 03 patients with cystic VS developed CSF leak, 02 patients through the incision and were treated by a lumbar puncture for 10 days and one patient developed CSF rhinorrhea and required revision surgery and packing of the air cells with an autologous fat graft. One patient developed CSF leak through the incision and complicated meningitis which was complicated by hydrocephalus required external ventricular drainage. After 10 days of treatment meningoencephalitis appeared and the patient died 30 days after surgery.
The extent of tumor resection will be assessed, based on the postoperative MRI performed 1 month after surgery. Total resection was performed in 09 cases and in 03 cases total tumor resection was not possible and a thin layer of the tumor was left on the facial nerve (Figure 1 and Figure 2): Within the meatus in one case, and along the facial nerve in one case.
Cystic VS is currently considered a separate entity due to its clinic, its evolution and its postoperative morbidity.
The incidence of cystic vestibular schwannomas ranges from 5% to 48%, with recent reports suggesting the real incidence to be approximately 10% [5].
Cystic VS is a tumor characterized by the rapid onset of clinical signs, the frequency of signs of compression of the brain stem at the time of diagnosis, the postoperative morbidity related to the adhesion of the tumor capsule to the facial nerve and the frequency of hematomas of the operating cavity.
The mechanisms of cyst formation and its rapid evolutionary potential are not clear. Charabi, et al. [6,7] postulated 3 mechanisms of cyst formation in cystic VS. These were rapid tumor growth leading to central necrosis, repeated intratumoral hemorrhage [8] and the coalescence of the microcysts in Antoni type B tissue, forming a capsular structure. Recently, Moon, et al. [9] identified the matrix metalloproteinases within the cyst fluid and the walls. They suggested that these enzymes could contribute to cyst formation and adhesion of the tumor to the surrounding structures.
For many authors, surgery remains the most recommended treatment [5-17]. Due to its rapid evolutionary potential, surveillance is not recommended [18]. Concerning the effectiveness of radiosurgery, it remains controversial because of the sudden increase in size of the cystic portion after radiosurgery [3,5]. Lim, et al. [19] about a series of 24 cases of cystic VS treated by Gamma knife radiosurgery concluded that tumor control was observed in 18 cases. Six patients experienced tumor progression. Of those 06 patients, 02 underwent surgery and one underwent repeat radiosurgery.
For Piccirillo, et al. [3] three factors need to be considered before cystic VS resection: Thickness of the cyst wall, position of the cyst, and extrameatal size of the lesion, including cystic and solid components.
Our per-operative observation found that cystic VS with Peripheral and thin wall (type B PC) was more difficult to resect. In this form the thin cyst wall adheres strongly to the vessels, brainstem, and nerves in particular to the facial nerve, it makes creating a plane of cleavage very difficult. In 02 cases the identification of the facial nerve was not possible and in 2 cases a thin layer of tumor was left over the facial nerve to preserve its function. The same observations have been reported by Metwali [5] and Piccirillo [3].
We also found that dissection of the trigeminal nerve, in 04 cases was accompanied by severe bradycardia < 40 b/min. The heart rate returned spontaneously to normal levels with cessation of manipulation of the trigeminal nerve: It was the trigeminocardiac reflex that was first described by Schaller in 1999 [20] and since 2005 it has been a brainstem reflex [21].
The postoperative morbidity was marked by 4 major complications: hematoma of the operative cavity in 4 patients, worsening of the low nerve function in 2 patients, worsening of the facial nerve function in 4 patients and CSF leakage in 4 patients [22,23]. The incidence of postoperative hematomas was significantly greater with cystic vestibular schwannomas, it was 08% in Metwali, et al. study’s [5]. This complication is related to the matrix metalloproteinase II, which could play a role in increasing vascular fragility [9].
The deterioration of lower nerves function, according to our surgical observations, is related to a spasm of the PICA. In the 02 cases, the PICA lying between the thin cyst wall and its dissection causes arterial spasm which can explain this aggravation.
The facial nerve function in the early postoperative period had worsened in 03 cases in our study (Table 4). Fundova, et al. [10], Yashar, et al. Benech, et al. [24], Jian, et al. [18], Metwali, et al. [5], Tang, et al. [13], Xia, et al. [25] and Eser Ocak, et al. [26] have reported poorer facial nerve outcome in the immediate postoperative period. For all these authors the preservation of the facial nerve function in cystic VS was responsible for a non-total tumor resection (Table 5). Liang, et al. [27] published a meta-analysis studying the clinical and surgical particularities of CVS. They find that the rate of favorable outcome of facial nerve function was significantly lower in cystic VS. The worsening of the facial nerve function is explained by the strong adhesion of the facial nerve to the tumor capsule frequent in cystic VS type B (08 cases) and the non-identification of the facial nerve (02 cases) explained by the deformation of the relationship of the tumor with the facial nerve, after the cyst has been removed, making identification and dissection of the facial nerve difficult.
Table 5: Literature review of postoperative results of cystic VS surgery regarding quality of resection, anatomical preservation of the facial nerve (FN) and facial nerve function according to the House and Brackman classification HB. View Table 5
Some intraoperative prognostic factors can predict facial nerve function, particularly the threshold of response to facial nerve stimulation at the end of surgery [28]. Indeed, a low response < 240 µvolts to a facial nerve stimulation > 2 mA predicts a worsening of facial nerve function. In case of worsening of the facial nerve function, we perform an electromyogram 3 months after the surgery to look for signs of reinnervation. In case of total absence of signs of reinnervation, we propose to the patients a facio-hypoglossal anastomosis.
Cystic vestibular schwannoma is a particular entity of vestibular schwannomas, characterized by the frequency of signs of brainstem compression at the time of diagnosis, and by the high frequency of postoperative morbidity.
Postoperative complications are explained by severe adhesion of the tumor wall to the facial nerve conditioning its function, to the hemorrhagic character of the solid part of the tumor which explains the frequency of hematomas of the operating cavity which may be associated with hydrocephalus and to severe adhesion of the tumor to the PICA explaining its vasospasm responsible for the aggravation of the function of low nerves.