Background: Literature has shown that subcutaneous immunotherapy (SCIT) is time-consuming and costly for patients in Canadian and US settings. However, there is no evidence of the patient time-use and direct patient costs related to SCIT in a Spanish setting. The objective of this study was to investigate self-reported direct costs and time-use related to SCIT among both caregivers of children with allergic rhinitis (AR) and adults with AR in a Spanish setting. .
Methods: In this cross-sectional study, 286 adults and 513 caregivers of children with symptomatic AR of at least moderate severity who are currently or have previously received allergy immunotherapy (AIT) entered the questionnaire. We excluded respondents who partly completed the survey, who are receiving or have received AIT using a sublingual or an unknown route, or who stopped AIT more than five years earlier. 87 adults and 289 caregivers of children (aged 5-17) were included. An electronic survey was conducted to collect self-reported time-use and direct costs related to transportation associated with the participants' most recent SCIT visit. Univariate descriptive statistics were used to analyze data.
Results: Results showed that adults on SCIT spend a total of 60 minutes per clinic visit, whereas caregivers and children spend a total of 50 minutes per visit, including transportation time and time spent at the clinic. Most adults and accompanying adults did not uphold any direct costs related to either parking, road tolls or other transport-related costs when receiving SCIT. The majority of adults, caregivers and children missed work or school due to SCIT. We found that a median of 3 hours of work or school was missed per visit.
Conclusion: Our findings indicate that SCIT entails substantial time-use and productivity loss for adults with AR as well as caregivers of children with AR in Spain. Furthermore, we found that treatment with SCIT has a negative impact on the educational attendance among children with AR.
Allergy immunotherapy, Allergic rhinitis, Productivity loss, Time-use, SCIT
AIT: Allergy Immunotherapy; AR: Allergic Rhinitis; EAACI: European Academy of Allergy and Clinical Immunology; IgE: Immunoglobulin E; SCIT: Subcutaneous Immunotherapy; SLIT: Sublingual Immunotherapy; VAS: Visual Analogue Scale
Allergic rhinitis (AR) is an inflammatory disease induced by inhaled allergens. Symptoms occur when allergen molecules activate an inflammatory response in the nasal mucous membranes. The inflammatory response in AR is mediated by immunoglobulin E (IgE), and the categorization of AR is often perennial (eg: dust mites) or seasonal (eg: pollen) and can further be classified by symptom severity [1,2].
In Spain, an estimated 21.5% of adults have AR [3]. The mean prevalence of AR in 13-14-year-oldSpanish children was found to be 9.3% [4]. Furthermore, the sensitization pattern in patients has been investigated by Valero, et al. [5], who found that patients with AR in Portugal and Spain are most often sensitized to grass, dust mites and olive tree pollens [5].
AR is accompanied with nasal symptoms such as sneezing, itching, rhinorrhea, and/or nasal congestion [6]. Studies have found that AR negatively affects a person's concentration, work productivity, quality of life and sleep [7,8]. Hence, missed days of school or work as well as days with reduced activity as a consequence of AR have also been reported in the literature [9]. Additionally, AR is associated with numerous comorbid disorders such as conjunctivitis, allergic asthma and atopic dermatitis [2,7].
AR can be managed in different ways, including allergen avoidance, entailing educating the patient to better understand the connection between the exposure to allergens and symptoms, treatment with symptom-relieving medication, and allergy immunotherapy (AIT) [1,2]. While symptom-relieving medication, for example intranasal corticosteroids and antihistamines, is recommended as first-line therapy [2,7], AIT is recommended to patients with a confirmed IgE-mediated disease who are not well controlled on symptom-relieving medication [8,10,11]. Compared to treatment with symptom-relieving medication, AIT modifies the immune pathways that cause the allergic response. AIT products are not generic, but some products have proven to provide a long-term disease-modifying effect even after stopping therapy [12,13]. Results from the Spanish National Survey on Allergic Diseases (Alergológica 2015) showed that more than half of the adults and children had moderate or severe AR, and approximately 31.5% of the adults and children were treated with AIT [14,15].
AIT can either be administrated as subcutaneous immunotherapy (SCIT) or as sublingual immunotherapy (SLIT) (tablets or drops) [16]. However, SCIT is traditionally preferred among Spanish adults, resulting in SLIT-tablets being prescribed less often [17]. The National Survey on Allergic Diseases (Alergológica 2015) showed that SCIT was used for 85.5% of the adults and 77.8% of the children [14,15].
SCIT is recommended to be administered by a healthcare professional and to continue over a three-to-five-year period [18]. SCIT is divided into two phases. The first phase, also called the build-up phase, consists of injections which comprise increasingly larger amounts of allergens, normally distributed once a week over 2-3 months. The maintenance phase comprises injections every fourth to sixth week [2,19]. This results in between 8 and 13 visits at the clinic every year during the maintenance phase.
The starting dose in the build-up phase is 1,000 to 10,000 times lower compared to the second phase, the maintenance phase. However, the maintenance phase can be reached sooner by using an accelerated build-up (either rushed immunotherapy or cluster immunotherapy). When using a rushed schedule, the patient receives increasing doses at intervals of 15 to 60 minutes over one to three days until the maintenance dose is achieved. Another possibility is the cluster schedule, where the patient receives multiple shots at each visit over a period of four to eight weeks [10]. Due to a risk of a severe systemic allergic reaction also known as anaphylactic shock, it is recommended that patients are monitored for half an hour after injections [1,10].
No evidence of the patient time-use and direct patient costs related to SCIT in a Spanish setting was identified in the literature. Time-use has been investigated in Canadian and US settings [20]. Results showed that SCIT is time-consuming for patients, which also supports the fact that time used in relation to SCIT is the most cited reason for patients ending the treatment earlier than planned [21]. In addition to being time-consuming, lost earnings and travels expenses are examples of direct costs which might become burdensome for patients on SCIT.
The objective of this study was to investigate the self-reported direct costs and the time-use related to SCIT among adults with AR as well as caregivers of children with AR in Spain. The total patient costs related to SCIT are of importance for decision-makers working with reimbursement, regulatory approval or treatment guidelines.
The time-use and direct cost of SCIT were investigated using an electronic survey prepared in Spanish. The survey has previous been used in the US [22]. Respondents were asked to recall their last SCIT visit; hence, all results are self-reported. Respondents included were adults with AR and caregivers of children (aged 5-17) with AR who answered on behalf of the children. Respondents were invited if their AR was of at least moderate severity, as defined by the m-ARIA severity scale, and symptomatic. Furthermore, only respondents who currently were receiving or previously had received AIT were asked to answer the survey, though respondents who stopped their therapy earlier than 2015 were excluded. This was done to ensure precise recollection and avoid recall bias.
The survey included allergy-specific questions which were made to assess respondents' (or children's) symptoms, specific allergies and medication use. Furthermore, the survey included sociodemographic questions, determined whether respondents were diagnosed with asthma and evaluated their AIT course. Quality of life was measured using the visual analogue scale (VAS) and EQ-5D-5L.
Respondents were asked to think back on their latest SCIT when answering questions about direct costs and time consumption in relation to the treatment. Questions were made to specify the time spent on transportation to the clinic (one-way) and the mode of transport. Furthermore, respondents were asked about the total duration per visit and how long they remained at the clinic for observation after receiving SCIT. Other costs, as for example tolls, gasoline and parking, were also measured through the questionnaire. In cases where caregivers answered the questionnaire on behalf of a child, they were asked who accompanied the child to the clinic if the child was accompanied.
To measure productivity loss among respondents, they were asked whether they had experienced missing work due to receiving SCIT as well as the number of hours they missed. Caregivers were asked if they or another person who accompanied the child to the clinic missed any work due to the treatment, and whether the child missed hours at school.
The questionnaire was sent out in collaboration with Kantar/Gallup. Data were collected from October 27 to November 24, 2020.
Respondents who were receiving or had received SLIT were excluded from the study. The same applies to respondents who finished SCIT before 2015. We also excluded respondents who only partly completed the survey. Before statistical analyses, survey responses were validated by checking for consistency and errors in the answers.
Univariate descriptive statistics (medians, means, frequencies and SD) were used to analyze data. Results of the descriptive statistics are reported as means, while cost and time-use findings are reported as medians, since medians are less sensitive to outliers.
286 adults diagnosed with AR and 513 caregivers of children diagnosed with AR entered the questionnaire. Of those, 199 adults and 224 caregivers did not complete the questionnaire, had received SLIT, did not know if they had received SCIT or SLIT, or ended their AIT before 2015. Therefore, these respondents were excluded. In total, 87 adults and 289 caregivers were included in the statistical analyses. Figure 1 presents a flowchart of the inclusion process.
 Figure 1: Flowchart of the study population.
View Figure 1
Figure 1: Flowchart of the study population.
View Figure 1
Demographic characteristics of the study population are presented in Table 1. Among the adults diagnosed with AR, 56% were male, the mean age was 38 years, and the mean EQ-5D score was 0.819. The mean EQ-5D score of the subgroup with concomitant asthma was 0.806, and the mean EQ-5D score of the subgroup without asthma was 0.837. The mean age for caregivers was 39 years, and among children it was 11 years. 54% of the caregivers were male, while 67% of the children were male. For adults and caregivers, the median household income was between €20,000 to €29,999 and €30,000 to €39,999, respectively.
Table 1: Demographics. View Table 1
Disease-specific characteristics of the study population are presented in Table 2. In total, 65% of the included adults with AR had a concomitant asthma diagnosis. Among the children with AR, 44% also had an asthma diagnosis. Over 70% of both adults and children had more than one allergy. Most respondents in this study reported use of decongestants, oral antihistamines, eye drops, nasal sprays and drops.
Table 2: Disease-specific characteristics. View Table 2
The SCIT-related self-reported time-use and direct costs related to a clinic visit are shown in Figure 2. Only one child was not accompanied to the clinic by an adult. Of those accompanied by an adult, 85%, 26% and 2% of the children were accompanied by the caregiver answering the survey, the caregiver's partner or another adult, respectively. Some children were accompanied by more than one adult.
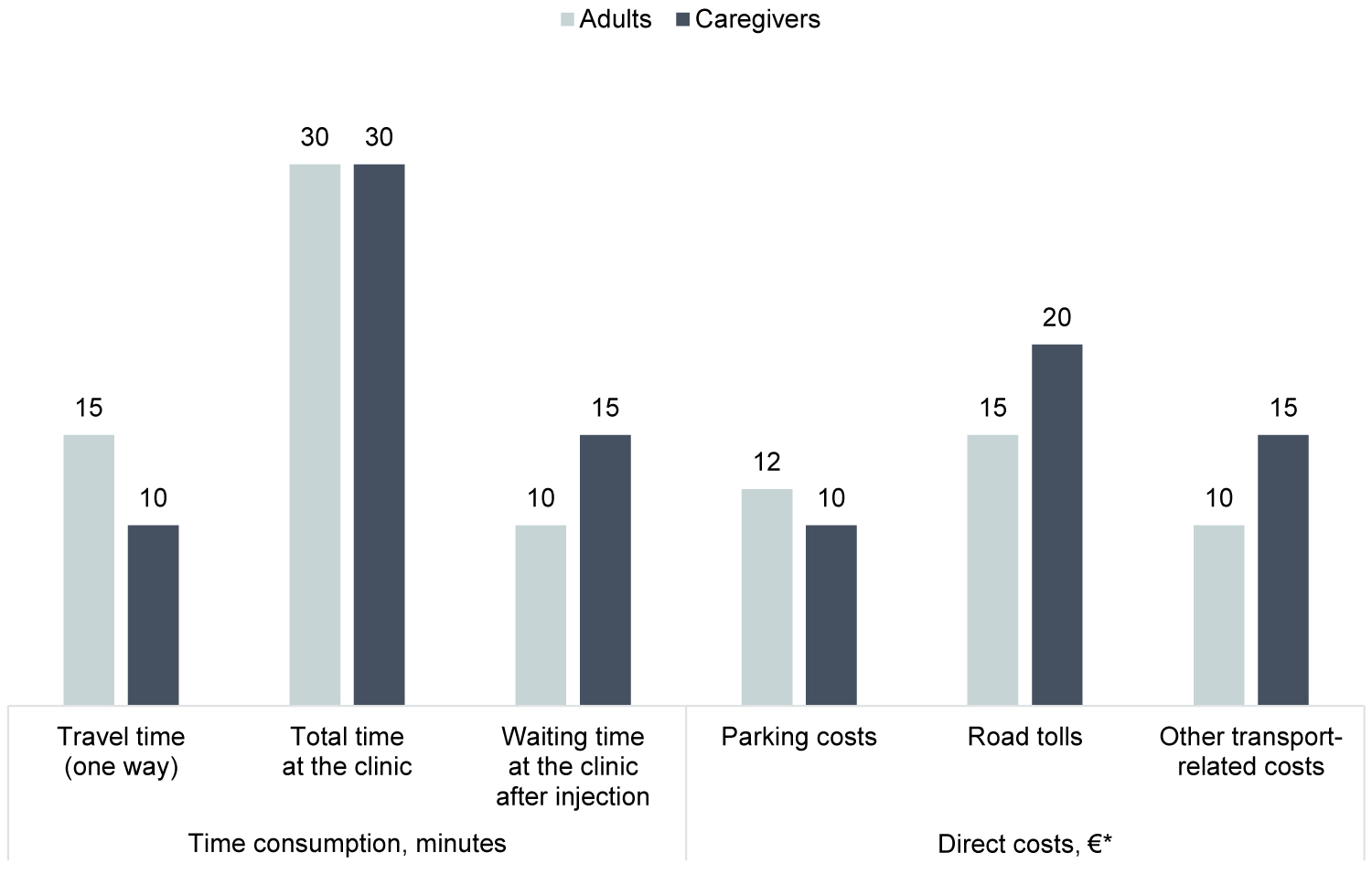 Figure 2: The time consumption and direct costs associated with one visit at the clinic for subcutaneous immunotherapy, median.
Figure 2: The time consumption and direct costs associated with one visit at the clinic for subcutaneous immunotherapy, median.
*Median direct costs are only illustrated for those of the population who upheld any costs in each category. Parking cost: Adults (N = 31), caregivers (N = 114). Road tolls: adults (N = 21), caregivers (N = 41). Other transport-related costs: Adults (N = 29), caregivers (N = 20).
View Figure 2
The median self-reported time spent on travelling one-way to the clinic was 15 minutes for adults and 10 minutes for caregivers with children, the median total time spent at the clinic was 30 minutes for both adults and caregivers with children, and the median waiting time after injection was 10 minutes for adults and 15 minutes for caregivers accompanying a child. In total, adults spent 60 minutes per clinic visit, whereas caregivers with children spent 50 minutes per clinic visit. The self-reported waiting time after administration of SCIT for both adults and caregivers with children is below the recommended 30 minutes [18].
The majority of both the adults and the caregivers did not uphold any direct transportation costs, i.e parking, road tolls or other transport-related costs (for example, gasoline or wear and tear of the car), related to a clinic visit. More specifically, 64%, 75% and 67% of adults with AR did not uphold any direct costs related to parking, road tolls or other transport-related costs, respectively. 60%, 85% and 68% of the caregivers of children with AR did not uphold any direct costs related to parking, road tolls or other transport-related costs, respectively. The median direct costs for the adults who upheld any costs related to a clinic visit was€12, €15 and €10 for parking, road tolls and other transport-related costs, respectively. The median direct costs for caregivers who upheld any costs related to a clinic visit was €10, €20 and €15 for parking, road tolls and other transport-related costs, respectively. This results in a total self-reported direct cost (median) per clinic visit of €37 for adults and €45 for caregivers who upheld any costs.
60% of adults either partly or completely missed work due to receiving SCIT. Adults accompanying a child to the clinic also often missed work either partly or completely. 70% of caregivers missed work, 59% of caregiver's partners missed work, and 25% of other adults missed work due to accompanying a child to receive SCIT. Both adults and adults accompanying a child miss a median of three hours from work due to one visit at the clinic. 48% of adults travelled to the clinic by car as either the driver or as a passenger. The equivalent number for adults accompanying a child was 89%.Furthermore, 98% of the children receiving SCIT go to school, and of those, 75% receive SCIT during school hours. Children receiving SCIT miss a median of three hours of school per visit at the clinic (Table 3).
Table 3: Lost productivity and mode of transportation related to SCIT. View Table 3
The objective of this study was to investigate self-reported direct costs and time-use related to SCIT among both caregivers of children with AR and adults with AR in a Spanish setting. Results showed that adults on SCIT spend a total of 60 minutes per visit to the clinic, whereas caregivers with children spend a total of 50 minutes per visit. This includes transportation time and the time spent at the clinic. The median self-reported waiting time after the injection was 15 minutes for adults and 10 minutes for children. The median self-reported waiting time for both groups was below the 30 minutes recommended by the European Academy of Allergy and Clinical Immunology (EAACI) [18]. The reason for not following the guidelines was not investigated in the survey. Several factors could result in the short waiting time, for example recall bias, as the respondents were asked to recall their last visit at the clinic, which could have happened within the last five years. The shorter waiting time could also be a result of respondents having received SCIT multiple times and never experienced an adverse event after treatment, and therefore, the waiting time was shortened. Blume, et al. [20] investigated the time-use in Canadian and US settings and reported that two-thirds of the clinics required that the patient waited in the clinic for a minimum of 30 minutes after the injection. Even though the clinical setting in Spain is not necessarily identical to those of Canada and the US, it is not unlikely that the short waiting time in this study is a result of the clinics in Spain not requiring patients to stay for minimum 30 minutes as seen in Canada and the US.
Blume, et al. [20] also reported that the total time spent per clinic visit to receive SCIT was an average of 80 minutes, which is higher than the total median time reported in this study, i.e 60 minutes for adults and 50 minutes for caregivers with children.
In Spain, most of the adults receiving SCIT and most caregivers of children receiving SCIT did not uphold any direct costs related to parking, road tolls or other transport-related costs, resulting in the median cost for all three cost categories being €0. Of those respondents for whom the clinic visit entailed any direct costs, the median was €12, €15 and €10 for parking, road tolls and other transport-related costs for the adults, respectively. The equivalent results for caregivers of children receiving SCIT was €10, €20 and €15 for parking, road tolls and other transport-related costs, respectively.
The self-reported median time missed at work was three hours per clinic visit for both adults receiving SCIT and adults accompanying a child to receive SCIT.59% of adults and 67% of caregivers missed work due to receiving treatment. With an average hourly wage in Spain of €14.58 [23], the productivity loss in Spanish adults and caregivers is around €1,575 per full treatment course of SCIT, if assuming a dosing schedule of one injection per month over three years. If 22% of Spanish adults between 18 and 65 years have AR, 31% of those are receiving AIT, and 86% of those receiving AIT are receiving SCIT, approximately 1,750,250 adults of working age (18-65 years) are receiving SCIT in Spain. If assuming all of these patients complete the three-year treatment course, these patients incur a total annual productivity loss of €542 million. Similarly, if assuming a 9% prevalence of AR in Spanish children, of those, 32% are receiving AIT, and of those, 78% are on SCIT, approximately 181,200 children in Spain are receiving SCIT. This results in an annual productivity loss for caregivers of €64 million, if assuming all of the children complete the full three-year treatment course. An alternative to receiving AIT as subcutaneous injections is SLIT-tablets, which are administrated sublingually. The advantage of SLIT-tablets is that patients can self-administer their treatment, as SLIT-tablets are taken at home by the patient themselves, except for the first dose, which is administered in a clinical setting in order to observe whether the patient experiences any serious adverse events [18]. Therefore, SLIT-tablets have the potential to reduce the number of necessary visits at the clinic and thereby reduce the productivity loss related to receiving SCIT. For some patients, it could also reduce the direct costs related to parking, road tolls and other transportation-related costs.
This study revealed that adults with AR have a lower quality of life compared with previously published quality-of-life estimates for the general Spanish population. The quality of life of the general Spanish population in the age group 18-79 years is 0.92 (weighted average of age groups) [24]. The quality of life for the adult respondents was 0.819 measured by EQ-5D-5L, which is 0.10 utility points below the utility score for the general Spanish population. The subgroup of adults with concomitant asthma had an even lower utility score of 0.806. This indicates the importance of treating patients with AR, especially patients with asthma and AR and can explain the patients' willingness to invest substantial time in receiving treatment.
A similar study investigated the share of adults and children with AR that also had asthma in Spain. The respondents in the study had never received AIT [Manuscript in preparation]. Results showed that 17% of the adults and 19% of the children with AR also had asthma. This is in contrast to findings in this study, where 60% of the adults and 44% of the children had asthma. Also, [Manuscript in preparation] reported that 45% and 56% of the adults and children, respectively, were polyallergic. In this study, 71% of the adults and 76% of the children were polyallergic. This indicates that AIT is more often prescribed to patients with a higher disease burden.
Due to the inclusion of respondents who received treatment within the last five years, answers in the questionnaire might be affected by recall bias. Respondents were asked to recall their last treatment at the clinic, which might have caused inaccurate answers.
In this study, 56% of the adult respondents were male. This differs slightly from results from a large retrospective cohort study from Germany, where 48% of the adults on SCIT were male. We do not believe that the difference in the gender composition affects the results of the study, as time consumption and direct costs related to transportation are gender-neutral.
The results of this study indicate that SCIT entails substantial time consumption for adults with AR as well as caregivers of children with AR and children with AR. Furthermore, a full treatment course with SCIT entails substantial productivity loss and negatively impacts the educational attendance of children with AR.
The survey was approved by Integ Review Institutional Review Board, Texas, US (Protocol number: 2258, approved in April 2020). Informed consent was obtained from all respondents before starting the survey. They were fully anonymous and could leave the survey at any time. The study was conducted in accordance with the Declaration of Helsinki.
Participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
This study was supported by ALK, Denmark. Ana Rosado Ingelmo has received fees from ALK for advisory services, and has participated in meetings from ALK, Novartis, Astra Zeneca and GSK. Jose MarÍa AusÍn Ruiz has received fees from ALK for advisory services. Adolfo GalánVivó, Mark Aagren and Henrik Brandi are employees at ALK. Mette Bøgelund, Mikkel Hasse Pedersen, Anna Okkels, and Anne Sofie Ledgaard Loftager are employees at Incentive Denmark, which is a paid vendor of ALK, Denmark.
All authors have made a significant contribution to the work whether in conception and design; analysis and interpretation of data; drafting/revision of the manuscript; revising it critically for intellectual content; or in all these areas. All authors have approved the final version to be published and are accountable for all aspects of the work.