To evaluate the efficacy of high frequency oscillatory ventilation (HFOV) in management of acute respiratory distress syndrome and determine whether if there is any superiority over the conventional mechanical ventilation (CMV).
A systematic review and meta-analysis were conducted according to PRISMA checklist and the Cochrane Handbook for Systematic Reviews of Interventions. The search of the literature was performed through several search databases: PubMed, MEDLINE via Ovid, and web of science.
We assessed the eligibility of all the relevant studies based on our inclusion and exclusion criteria. The methodological index for nonrandomized studies (MINORS) was used to assess the quality of the included studies. The quality and reliability assessment of the included studies were analyzed and appraised Quality Appraisal of Reliability Studies (QAREL) checklist.
Meta-analysis was conducted to compare CMV and HFOV in term of in hospital or 30-days mortality, therapy failure, ventilation related complications, and change in PaCO2 and PaO2:FiO2 ratio.
Twenty studies, involving 2153 patients, were eligible. 59.4% of the included patients received HFOV for management of acute respiratory distress syndrome. The mean age of the included patients was 52.2-years-old. The mortality rate of 45.9% was reported in HFOV group while 46.5% in CMV group. HFOV was associated with increase in risk of therapy failure and hemodynamically instability, with 17% and 53%, respectively. There was a slight decline in quality MINORS total score over the publication years (r = 0.00048, P = 0.94, CI = -0.6908 to 0.6516). Meta-analysis revealed that High frequency oscillatory ventilation would have the same risk of mortality as compared to conventional mechanical ventilation. (RR = 0.93, 95% CI: 0.71 to 1.21, P = 0.60).
High Frequency Oscillatory ventilation showed increase risk of therapy failure, when compared to CMV. However, there was not any significant difference between the two groups in term of mortality rate.
High frequency, Acute respiratory distress syndrome, Mechanical ventilation, Adult
Acute respiratory distress syndrome (ARDS) is a potentially life-threatening complication that usually occurs in critically ill patients. ARDS is characterized by diffuse lung inflammation with reduced lung compliance and impaired gas exchange [1]. ARDS' admission rate in intensive care unit (ICU) beds has experienced an increasing trend over the past few years. There is also a parallel alarming rise in mortality for patients with ARDS. A recent observational study including demonstrated that admission and mortality rate of ARDS in 50 different countries [2]. Out of 29,144 patients, 10.4% have fulfilled ARDS criteria to be admitted in ICUs, while the mortality associated with mild, moderate, and severe ARDS was 34.9%, 40.3%, and 46.1%, respectively [2]. In addition, 23% of admitted patients received mechanical ventilation as a line of management [2].
Mechanical ventilation is considered a pillar in ARDS management and outcomes. However, it can induce a further lung tissue injury paradoxically. This flip-side effect is called ventilator induced lung injury as result of mechanical disruption of alveolar-lining epithelium and capillary endothelium [3]. In addition, the associated release of inflammatory cytokines that may lead to ARDS exacerbation as well as multiple organ failure [4,5]. The incidence of ventilation induced lung injury is substantially increasing 25% of mechanically ventilated patients develop some sort of lung injury or even ARDS [6].
As such, there has been a recent heightened focus on implementing lung protective strategies to limit the alveolar mechanical over-distention and prevent its related clinical complication. All these strategies aimed to lower tidal volume and/or high end-expiratory pressures [7]. High frequency oscillatory ventilation (HFOV) provides smaller tidal volumes to limit the alveolar over-distension. It also maintains the alveolar recruitment and prevents the end-expiratory collapse. The increased popularity of HFOV stems from its ability to achieve the lung protective strategies [8,9].
The purpose of this manuscript is to systemically review available clinical trials to determine the feasibility, effectiveness, and impact of HFOV when compared to the conventional mechanical ventilation on increasing patients' survival, clinical improvement, and reducing ventilation related complications. As there are currently no definite guidelines for clinical applicability of this novel line of intervention, we also aim to provide recommendations and initial guidelines through the assessment and qualitative analysis of the available evidence.
The study protocol was conducted according to the PRISMA (Preferred Reporting Items for Systematic Review and Meta-analyses) statement and fulfilled the quality guidelines as reported in Cochrane Handbook for Systematic Reviews of Intervention [10].
A detailed comprehensive search for all relevant studies was conducted through PubMed, MEDLINE via Ovid, and web of science. In addition, to ensure inclusion of all available relevant evidence, we also manually searched the references of previous studies for any studies that meet our inclusion criteria. The Boolean operations and keywords used for the PubMed search were; (("Respiratory Distress Syndrome, Adult"[Mesh] OR "Acute Chest Syndrome"[Mesh]) OR "Respiratory Insufficiency"[Mesh]) AND ("High-Frequency Ventilation"[Mesh] OR "High-Frequency Jet Ventilation"[Mesh]). The search strategy was a combination of subject headings and free text words in Ovid MEDLINE, topic searching in Web of Science Figure 1. Flow chart showing study identification, inclusion, and exclusion of the studies.
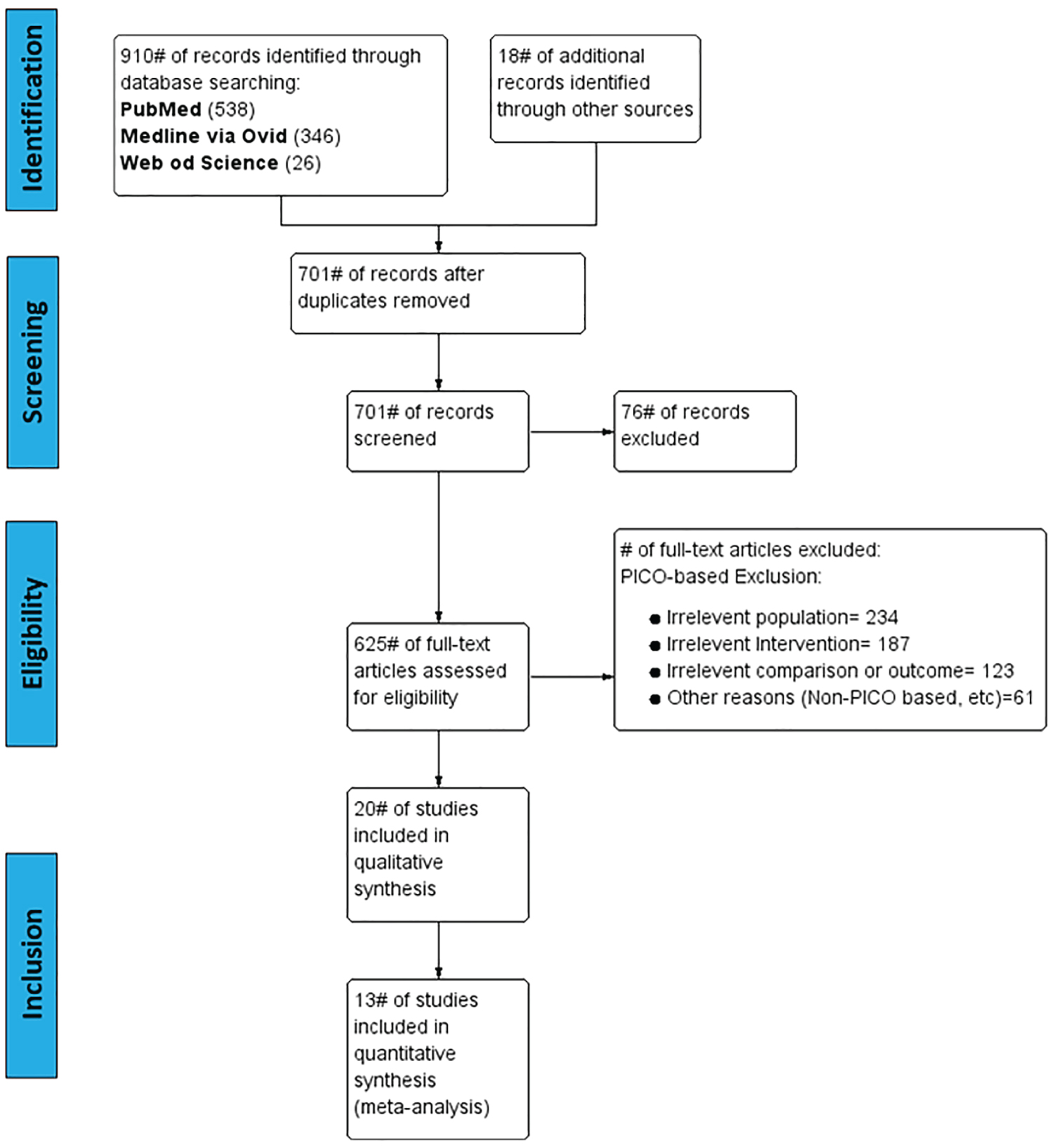 Figure 1: Flow chart showing study identification, inclusion, and exclusion. View Figure 1
Figure 1: Flow chart showing study identification, inclusion, and exclusion. View Figure 1
We assessed the eligibility of all the relevant studies based on our inclusion and exclusion criteria. Inclusion and Exclusion criteria were derived from PICO [Population, Intervention, Comparison and Outcome] question. In addition to, other Non-PICO based exclusion taxonomy [e.g. language, duplicate data/study, or data unavailability]. Two independent reviewers examined and analyzed article titles, article abstracts, and full-text documents for eligibility criteria.
1. Type of studies: Randomized controlled trials, cohort observational studies, non-randomized clinical trials, case series, and case reports published in English language investigating the use and efficacy of HFOV, as opposed to the use of conventional ventilation were considered for inclusion.
2. Type of participants: An eligible study should include adult patients who are suffering from ARDS who were receiving mechanical ventilation as a therapeutic line.
3. Type of interventions: The included studies must investigate the feasibility and efficacy of HFOV, either alone or compared to conventional mechanical ventilation to achieve better clinical and physiological outcomes with less adverse events.
4. Type of comparison and outcomes measures: Outcome analysis must evaluate the incidence of In Hospital or 30-day mortality, therapy failure, and HFOV-related complications. In addition, length of both mechanical ventilation and hospital stay between HFOV and conventional mechanical ventilation (CMV) groups.
Two authors extracted the data from the included studies independently. Self-designed tables were designed to sort both the qualitative and quantitative data for our analysis. The extracted data variables used were the following; 1) Demographics and study characteristics (Author, country of trial, year of Publication, Enrollment years, Type of Study, Study setting, and level of Evidence); 2) Patient characteristics (Patients numbers per each group, Mean Age); 3) ARDS enrollment criteria (Mean PaO2/FiO2, Oxygenation index [OI], Ventilator days prior study, and APACHE II score); 4) Ventilator settings; in HFOV group (Mean pressure, Mean frequency, and Amplitude) and in CMV group (Mode, tidal volume, and PEEP); 5) Outcome variables required for meta-analysis such as mortality, length of mechanical ventilation, incidence of complications, length of Hospital stay; 6) Changes before and after HFOV in terms of PaCO2 and PaO2:FiO2 ratio.
We used the Cochrane collaboration's assessment tool for risk of bias from Cochrane handbook for systematic review [10] to assess the methodological bias of included randomized controlled trials. The following items included the randomization, allocation concealment, blinding, incomplete outcome data (attrition bias), and selective reporting (reporting bias).
Two reviewers used the methodological index for nonrandomized studies (MINORS) [11] to evaluate independently the methodological quality of the included non-randomized trials. This index includes twelve items and each item was scored as "0" (not reported), "1" (reported but inadequate), or "2" (reported and adequate).
Quality Appraisal of Reliability Studies (QAREL) checklist [12] of 11 items provides a quality assessment for the spectrum of both subjects and examiners; examiner blinding, order effects of examination, suitability of the time interval among measurements, appropriate interpretation, and appropriate statistical analysis.
The primary outcomes assessed in this meta-analysis are in hospital or 30-days mortality, therapy failure, ventilation related complications, and change in PaCO2 and PaO2:FiO2 ratio. The secondary outcomes are length of ventilation dependency, and length of hospital stay (LOHS).
1. Meta-analysis of in hospital or 30 days mortality: Effect size was estimated when compared HFOV to CMV in 7 studies.
2. Meta-analysis of therapy failure: Effect size was estimated when compared HFOV to CMV in 4 studies.
3. Meta-analysis of ventilation related complications:
a. The incidence of Barotrauma: Effect size was estimated for the comparison between HFOV and CMV in 7 clinical trials.
b. The incidence of hemodynamically instability: Effect size was estimated for 6 studies that compared the incidence in HFOV and CMV group.
c. The incidence of endotracheal tube obstruction: Effect size was estimated for 3 studies that compared HFOV group to CMV group.
d. The incidence of refractory hypoxemia: Effect size was estimated when compared HFOV to CMV group in 4 clinical studies.
4. Meta-analysis of change in PaO2:FiO2 ratio: Effect size was estimated for the change in PaO2:FiO2 ratio before and after HFOV in 7 studies.
5. Meta-analysis of change in PaCO2: Effect size was estimated in 5 studies comparing PaCO2 value before and after HFOV.
1. Meta-analysis of Length of hospital stay (LOHS): There were only 3 studies with sufficient data to perform meta-analysis and estimate the effect size.
2. Meta-analysis of Length of ventilation dependency: Effect size was estimated for 6 studies comparing HFOV and CMV.
SPSS software (version 20.0.0; SPSS, Chicago, Illinois) was used to analyze the data. Both parametric (mean and standard deviation) and nonparametric (median and interquartile range) descriptive statistics were used. The Pearson correlation was used for normally distributed data.
Based on our search strategy, 1308 publication records were initially identified. In addition, 18 records were identified through manual references retrieving. After removal of duplicates, 991 records were screened based on the inclusion criteria. After initial screening, 366 studies were excluded based on the title and abstract. 605 studies were excluded after applying the PICO based- and non-PICO exclusion criteria on the full-text studies. The study selection process ended up with 20 included studies. Among these 20 clinical trials, nine were randomized controlled, seven were observational cohort studies, and four retrospective studies Figure 1.
Among the 2153 patients admitted for mechanical ventilation, 1281 patient in 20 studies received high frequency oscillatory ventilation, while 872 patients were being ventilated by CMV. The mean age of patients across the included studies was 52.2-years-old ranged from 37-81.7 years. Most of the included trials defined the criteria of ARDS enrollment. The baseline mean PaO2/FiO2 ratio in seventeen studies was 100.8, ranged from 52-139. Besides, the mean oxygenation index across 11 studies was 30.6, ranged from 19.2-52. In addition, mean score of Acute Physiology and Chronic Health Evaluation (APACHE II) was 24.3, ranged from 17-35. Sixteen studies have reported the duration of being ventilated conventionally before HFOV. The mean CMV ventilation days prior HFOV was 3.5 days, ranged from 0.5-7.7 days. Table 1 includes the characteristics of patients and intervention settings.
Table 1: Patients and intervention characteristics. View Table 1
Out of twenty included studies, seven studies [13-19] compared CMV and HFOV about the mortality rate. The mortality rate was reported in both CMV and HFOV groups as 46.5% and 45.9%, respectively. Most of studies define the failure of the treatment as persisting low oxygenation profile or even refractory hypoxemia after being ventilated. Comparing the risk of therapy failure between CMV and HFOV, 17% and 11% were reported in [13-16] reported as the percentage of patients failed to achieve therapy goals. Ventilation complications were presented as barotrauma, hemodynamically instability, refractory hypoxemia, or endotracheal tube obstruction. Seven studies compared the incidence of barotrauma due to high airway pressure during mechanical ventilation. Their results showed 6% and 7% of risk in CMV and HFOV, respectively. HFOV was associated with 53% of hemodynamically instability, while 48% was demonstrated in CMV group. In term of refractory hypoxemia, CMV was associated with a higher risk than HFOV with 11% and 6%, respectively.
Although all the included RCTs reported a random sequence generation and allocation concealment, either briefly or in details, Bollen, et al. [13], Ferguson, et al. [15], and Mentzelopoulos, et al. [17] showed high performance and detection biases. There were no any attrition bias or incomplete data outcome in almost all the included trials except of Bollen, et al. [13] Review authors' judgements about each risk of bias item for each included study are described in (Figure 2A and Figure 2B).
 Figure 2: (A) Risk of bias graph: Review authors' judgements about each risk of bias item presented as percentages; (B) Across all included studies and for each included study; (C) Total methodological index for non-randomized studies (MINORS) score for the included studies plotted against publication year. View Figure 2
Figure 2: (A) Risk of bias graph: Review authors' judgements about each risk of bias item presented as percentages; (B) Across all included studies and for each included study; (C) Total methodological index for non-randomized studies (MINORS) score for the included studies plotted against publication year. View Figure 2
There was a slight decline in MINORS total score over the publication (r = 0.00048, P = 0.94, CI = -0.6908 to 0.6516). The R2 value suggests that 0.04% of MINORS decline over time could be predicted by year of study publication (Figure 2C). Most studies had methodological limitations, with an average MINORS score of 10.36 ± 3.9, ranged from 3-15 (Table 2).
Table 2: Methodological Items for non-randomized studies (MINORS) score distribution. View Table 2
Most of the studies have low methodological scores in the following items:
1. Inclusion of consecutive patients.
2. Unbiased assessment of the study endpoint- objective and subjective evaluations were not blinded or the reasons for blinding were not stated.
3. Prospective calculation of the study size.
The QAREL checklist was used to appraise the reliability analysis of the included clinical trials. Among the twenty studies, five demonstrated high quality, one study was of moderate index, and thirteen studies were of low index on quality appraisal. The reliability's range of the included studies was between 3 and 10, out of a total score of eleven (Table 3).
Table 3: Assessment based on Quality Appraisal of Reliability Studies (QAREL) checklist. View Table 3
1. Meta-analysis of in hospital or 30 days mortality
Six studies [13-19] -including 1705 patients- reported the data of incidence of mortality. There was statistically significant heterogeneity in the studies (P = 0.001; I2 = 75%). Using Random effect model, the outcome results revealed that High frequency oscillatory ventilation would have the same risk of mortality as compared to conventional mechanical ventilation. (RR = 0.93, 95% CI: 0.71 to 1.21, P = 0.60) Figure 3A.
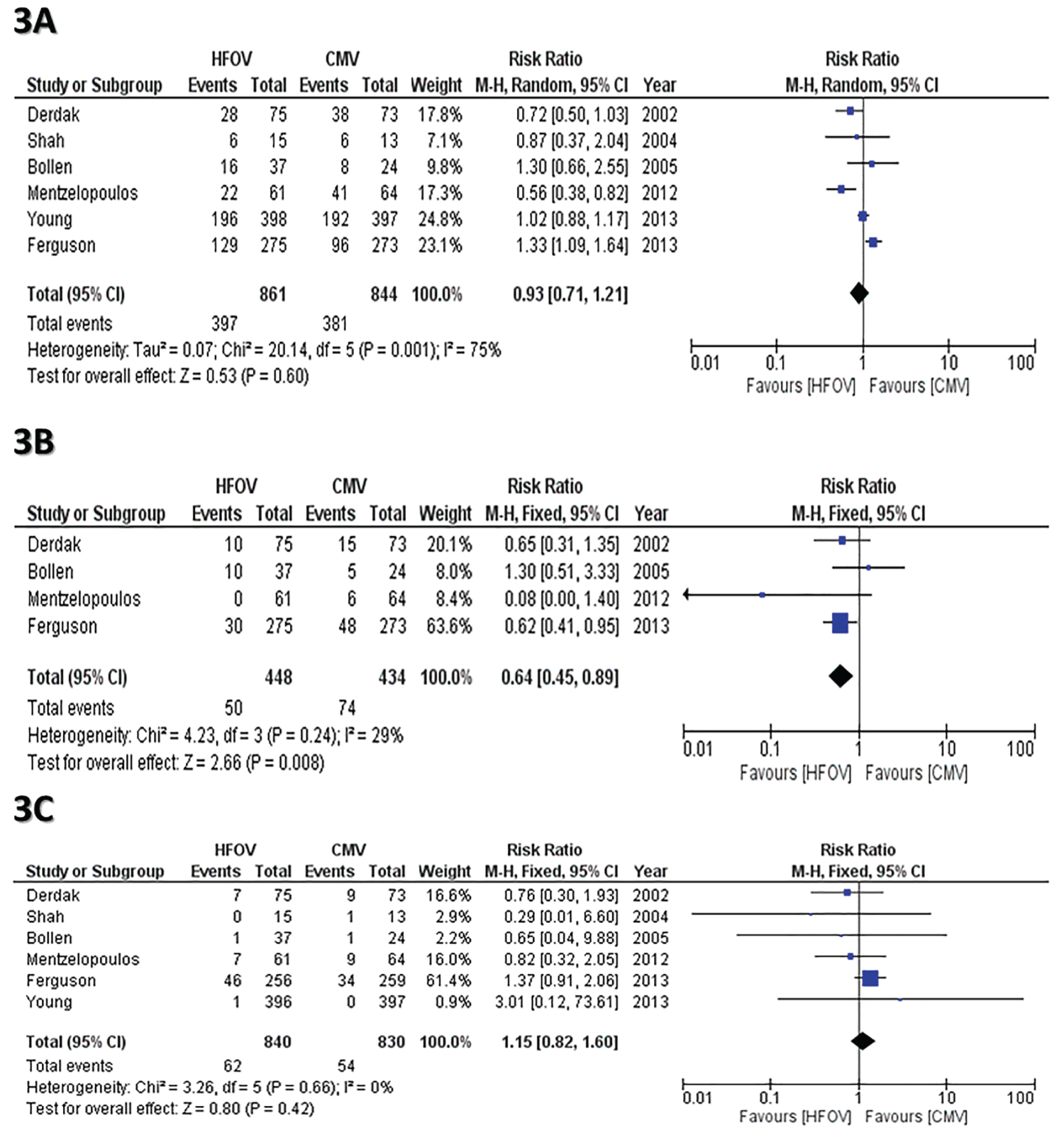 Figure 3: (A) Forest plot the incidence of in hospital or 30 days mortality between CMV and HFOV; (B) Forest plot compares Risk of therapy failure between CMV and HFOV; (C) Forest plots compares Risk of HFOV complications [Barotrauma]. View Figure 3
Figure 3: (A) Forest plot the incidence of in hospital or 30 days mortality between CMV and HFOV; (B) Forest plot compares Risk of therapy failure between CMV and HFOV; (C) Forest plots compares Risk of HFOV complications [Barotrauma]. View Figure 3
2. Meta-analysis of therapy failure
The therapy failure rate was provided in 4 studies [13-18]. There is no statistically significant heterogeneity in the included studies (P = 0.24; I2 = 29%). Using fixed model effect, the outcome showed that CMV has a higher risk in therapy failure as compared to HFOV (RR = 0.64, 95% CI: 0.45 to 0.89, P = 0.008) Figure 3B.
3. Meta-analysis of ventilation related complications
a. The incidence of Barotrauma: Six studies [13-19] reported the data of incidence of barotrauma as a ventilation complication. There was no statistically significant heterogeneity in the studies (P = 0.66, I2 = 0%). When the fixed-effect model used, the results indicated that there is no statistical significance between both groups in terms of the incidence of barotraumas (RR = 1.15, 95% CI: 0.82 to 1.60, P = 0.42) (Figure 3C).
b. The incidence of hemodynamically instability: Six studies [13-19] reported the data of incidence of being hemodynamically unstable as a ventilation complication. There was a significant heterogeneity in the studies (P < 0.0001, I2 = 81%). With the utilization of random-effect model, the HFOV group show slight affinity for increased risk of unstable hemodynamics -without statistical significance- when compared to CMV group (RR = 1.08, 95% CI: 0.85 to 1.37, P = 0.53) (Figure 4A).
 Figure 4: (A) Forest plots compares Risk of HFOV complications [Hemodynamically instability]; (B) Endotracheal tube obstruction, and; (C) refractory hypoxemia] between CMV and HFOV; (D) Forest plots compares Change in (A) PaO2:FiO2 ratio. View Figure 4
Figure 4: (A) Forest plots compares Risk of HFOV complications [Hemodynamically instability]; (B) Endotracheal tube obstruction, and; (C) refractory hypoxemia] between CMV and HFOV; (D) Forest plots compares Change in (A) PaO2:FiO2 ratio. View Figure 4
c. The incidence of endotracheal tube obstruction: Only three clinical studies [14,17,20] reported the data of the incidence of endotracheal tube obstruction as a ventilation complication. There was no difference between HFOV and CMV. (RR = 1.30, 95% CI: 0.30 to 5.60, P = 0.73) (Figure 4B).
d. The incidence of refractory hypoxemia: The risk of refractory hypoxemia was reported in four studies [13-17]. There was no statistically significant heterogeneity in the studies (P = 047, I2 = 0%). Using the fixed effect model, CMV demonstrated higher risk of refractory hypoxemia as compared to HFOV. (RR = 0.60, 95% CI: 0.39 to 0.93, P = 0.02) (Figure 4C).
4. Meta-analysis of change in PaO2:FiO2 ratio: Seven studies [21-26] reported the data of PaO2:FiO2 ratio before and after being on High frequency oscillatory ventilation. There was statistical significance heterogeneity in the studies (P < 0.00001, I2 = 94%). Using random effect model, the results showed that HFOV induces a significant improvement in term of PaO2:FiO2 ratio (Std.MD = 1.62, 95% CI: 0.73 to 2.51, P = 0.0003) (Figure 4D).
5. Meta-analysis of change in PaCO2: Five studies [21,23-26] reported the data of PaCO2 before and after being on High frequency oscillatory ventilation. There was statistical significance heterogeneity in the studies (P = 0.008, I2 = 71%). Using fixed effect model, the results showed that HFOV induces a significant improvement in term of PaCO2. (MD = -4.17, 95% CI: -6.95 to -1.40, P = 0. 003) Figure 5A.
 Figure 5) Forest plots compares (A) PaCO2 before and after HFOV; (B) The length of mechanical ventilation; (C) Length of Hospital stay (LOHS) between CMV and HFOV. View Figure 5
Figure 5) Forest plots compares (A) PaCO2 before and after HFOV; (B) The length of mechanical ventilation; (C) Length of Hospital stay (LOHS) between CMV and HFOV. View Figure 5
1. Meta-analysis of Length of ventilation dependency: The length of ventilation dependency was reported in five studies [13-17].There was no statistically significant heterogeneity in the studies (P = 0.95, I2 = 0%). Using fixed effect model, the results showed that patients who are on HFOV would have longer time (1.28 days) on ventilation, when compared to CMV (MD = 1.28, 95% CI: 0.17 to 2.38, P = 0.02) Figure 5B.
2. Meta-analysis of Length of hospital stay (LOHS): Three studies [15-17] reported the length of hospital stay in HFOV and CMV groups. There was no statistically significant heterogeneity in the studies (P = 0.83, I2 = 0%). There was no difference between HFOV and CMV (RR = 1.24, 95% CI: - 0.08 to 2.56, P = 0.07) Figure 5C.
This is the largest meta-analysis pooling 2153 patients to determine the superiority and compare HFOV to CMV regarding supporting the patients' survival and reducing the ventilation-related complications. This study also provides a comprehensive methodological quality's assessment of both randomized and non-randomized trials. Our study's methodology aimed to minimize any bias through performing a comprehensive search of the available literature, defining our clear aims and hypotheses as well as evaluating the main clinical and physiological outcomes for analysis.
The most featured finding of our meta-analysis was that HFOV has the superiority in significantly improving the oxygenation profile of the ICU admitted patients, when compared to their profile before ventilation. In addition, CMV was associated with higher risk for refractory hypoxemia and therapy failure than HFOV. In the term of the incidence of in-hospital mortality, both HFOV and CMV have the same risk without any associated superiority of either. However, HFOV is associated with increased risk of inducing hemodynamically instability such as hypotension.
Considering HFOV as a "Substitute" or an "Alternative" for CMV has been a major subject for an ongoing debate. Some investigators would consider HFOV as a substitute for CMV that provides the same functionality, just in a different form [27]. While others support that HFOV would be an alternative for better functions and outcomes that serve the same purpose [28]. These points of view have been built based on some studies' results showed that there is either equality or any superiority of HFOV in term of patient's survival, treatment success, physiological parameters or even healthcare logistics such as length of hospital stay and ICU admission [28-30].
HFOV has several beneficial advantages, such as lowering the tidal volume of ventilation therapy which minimizes the sheer stress over the alveolar wall, as well as maximizing lung recruitment [31-33]. These potential benefits should have provided a sensible reduction in patient's mortality. Mortality was determined as either at in-hospital [15-18] or at 30 days after randomization [13,14,19].
There is ongoing controversy about this potential efficacy of HFOV in improving the patient's survival. Mentzelopoulos, et al. [17] and his research group demonstrated that HFOV significantly reduce the mortality rate when they used it intermittently and for variable period over the day. On the other hand, Ferguson, et al. [15] showed that HFOV may increase the mortality if it's been early applied in ARDS patients. However, our meta-analysis, as well as the previous meta-analyses, showed that HFOV did not reduce in-hospital or 30-day mortality. In other words, there is no superior mortality benefit over the conventional ventilation [28,34]. There is still a limitation in evidence-based reporting of the primary outcome in the included studies. This risk of bias may affect the certainty of the conclusion about HFOV efficacy.
The hemodynamic effects of HFOV have also been investigated and reported by several clinical and animal studies. Derdak, et al. [14] demonstrated that there are no any differences between HFOV and CMV group in the regards of cardiac output, heart rate, and arterial blood pressure. In contrast, Fort, et al. [35] and David, et al. [23] presented that there was associated initial increase in central venous pressure and pulmonary artery pressure. In addition, Mehta, et al. [26] also reported reduction in cardiac output after HFOV application. Our analysis pooling the patients from several studies found that HFOV may have a slight affinity for increased risk of unstable hemodynamics -without statistical significance- when compared to CMV group (RR = 1.08, 95% CI: 0.85 to 1.37, P = 0.53). This result is consistent with the mechanism of HFOV's action. As HFOV induces increase in mPaw that would lead to increase in pleural pressure as well as decrease in venous return and cardiac output [36].
HFOV would not be suitable and efficient in all ARDS patients, as there are several variables that may interfere with the functionality of HFOV. One of these variables is the application timing. Most of the included studies considered the duration of conventional ventilation prior initiating HFOV as a potential variable that may influence the mortality incidence [18,23,26,35]. Our analysis showed there is wide range and heterogeneity in this duration among the trials, ranged from 0.5-7.7 days. In 2006, Bollen and his colleagues [29] demonstrated that this duration was significantly different between non-survivors and survivors. However, they couldn't find a significant association between prolonged CMV before HFOV initiation and the mortality rate.
Furthermore, the patient's positioning would have an impact on the therapy success and mortality rate. The comparison between prone and supine HFOV in term of oxygenation profile has been investigated by Papazian, et al. [37]. They concluded that patients in supine HFOV position have experienced much less improvement, when compared to prone HFOV and prone CMV. While other researches [14,26] have sharply contrasted this finding through just applying a little higher mPaw > 30 cmH2O to their studies' participants. The etiology of ARDS could be another potential variable. As the patients with extra-pulmonary cause of ARDS may significantly experience better improvement in oxygenation profile, when compared to pulmonary ARDS [38]. In addition, adjusting the age, APACHE II score, pH, and oxygenation index of the included patients' prior outcomes assessment would provide us with a better and clear conclusion about the effectiveness of HFOV.
Analyzing the quality of the included RCTs, we found that the blinding of outcome assessors was unclear which ended up with performance and detection biases. The blinding of professional who apply the ventilation support could be unfeasible. However, the blinding of outcome assessors can be easily done to avoid any detection bias. The overall methodological quality of the RCTs was acceptable. However, there is a slight decline in the methodological quality of non-randomized trials over the last decade. Further randomized controlled trials -with better quality, larger sample size, with clear hypotheses including adjusting of the confounding variables- are still required.
Our study was not exempted from some limitations. However, most of these are inherent to the methodology of the included studies. First, there is significant heterogeneity among the studies which may affect the conclusion such as the mortality reporting, defining therapy failure, mean age, APACHE II score and other baseline characteristics of both patients and intervention. This is kind of variability would make each patient condition unique. In addition, the overall sample size was relatively variable across the studies, which may influence of the estimation of the effect size. Despite the limitations, our study provides an updated evaluation not only for the outcome of HFOV in comparison to CMV, but also a detailed quality assessment for the available literature.
High Frequency Oscillatory ventilation showed increase risk of therapy failure, when compared to CMV. However, there was not any significant difference between the two groups in term of mortality rate. As well as there was no difference between HFOV and CMV in regard of ventilation related complications such as Barotrauma, refractory hypoxemia, and endotracheal insufficiency. While HFOV was associated with increase hemodynamically instability. On the laboratory aspect, HFOV revealed significant improvement of PaO2:FiO2 ratio and PaCO2.
Mohamed E Awad: Study design, data extraction, data analysis, data interpretation, writing the original draft.
Mohamed Gaber: Study design, data extraction, data interpretation, reviewing/editing the manuscript.
Suhib I Alhusban: Data interpretation, reviewing/editing the manuscript.
Sung Chu: Data interpretation, reviewing/editing the manuscript.
Colville HB Ferdinand: Study design, data interpretation, project administration and supervision, reviewing/editing the manuscript.
Mohamed Ben Omran: Study design, data interpretation, project administration and supervision, reviewing/editing the manuscript.
No additional funding sources were used for this article.
No conflict of interests is evident for the authors of this article.