Background: To evaluate risk factors for vaginal mesh exposure (VME) after mid-urethral sling (MUS) placement for urinary incontinence.
Methods: This is a case-controlled retrospective study at a tertiary care center. An institutional review board-approved database of women who underwent MUS sub-urethral excision procedure was reviewed. Demographic data, presenting symptoms, MUS placement technique, and location/size of exposure (none, < 1 cm, or > 1 cm) were collected for those who underwent MUS removal for symptomatic VME versus those with non-exposure indications. Risk factors were compared between those with VME and non-VME indications. For smoking, the effect size was estimated based on an age-matched, nationally reported dataset.
Results: Between 2005-2018, 496/499 patients were included. Vaginal mesh exposure cases were > 1 cm (56%) and more on the left (51%). Risk factors including obesity, hormone replacement therapy, diabetes, and smoking status were not associated with VME. Those with VME (n = 41) were younger and less likely than those without (n = 455) to have voiding dysfunction and urinary tract infections at presentation (p < 0.05).
Conclusion: Risk factors including obesity, hormone replacement therapy, diabetes, and smoking status in women who underwent MUS removal surgery were not associated with VME.
Exposure, Mesh, Midurethral sling, Risk factors, Smoking
SUI: Stress Urinary Incontinence; MUS: Mid-Urethral Sling; VME: Vaginal Mesh Exposure; SSR: Suburethral Sling Removal; HRT: Hormone Replacement Therapy; EPIC, Verona, Wisconsin: Electronic Medical Record; BMI: Body Mass Index; RUTIs: Recurrent Urinary Tract Infections; SUI: Stress Urinary Incontinence; UUI: Urgency Urinary Incontinence; MUI: Mixture of Both Urinary Incontinence
Stress urinary incontinence (SUI) is a common condition among women with prevalence estimates ranging from 4% to 42% [1,2]. Conservative measures such as Kegel exercises may offer relief for some women with SUI [3], and for those requiring surgical intervention, a mid-urethral sling (MUS) can be considered [4]. However, the rate of vaginal mesh exposure (VME) after MUS has been reported between 2-4% [5-8]. This unique MUS complication was recognized by the International Continence Society/International Urogynecological Association (ICS/IUGA) and prompted an international classification to report on size, location, and management of any mesh exposure [9]. More recently, a joint position statement by the American Urogynecologic Society and IUGA was released to analyze evidence concerning mesh complications and provide treatment recommendations; however, this position statement did not review causes or risk factors of VME [10].
Biologic and mechanical factors may contribute to VME after MUS placement. Low estrogen levels in the postmenopausal population as well as older age (> 70 years) can result in vaginal tissue thinning or atrophy, which can lead to VME. Weakened tissue may also result from obesity-related mechanical stress due to body habitus, and nutritional deficiencies, vascular insufficiencies, or immune mediator dysregulation may also contribute to the impaired wound healing associated with obesity [11]. Impaired wound healing has been observed among those with diabetes mellitus [12] as well as in smokers compared to non-smoking individuals [13-15]. However, the role of diabetes and smoking status in increasing the risk of VME after MUS has yielded conflicting results in the literature [5,6,16].
Our objective is to report data on referred women who underwent suburethral sling removal (SSR) surgery [17,18] after MUS placement because of either VME or non-VME indications. In these two cohorts (VME or non-VME indication), we evaluated if potential risk factors such as diabetes, menopause, hormone replacement therapy (HRT), obesity, and smoking status (compared to an age-matched general population) were associated with a greater incidence of VME compared to non-VME among those who underwent SSR.
A prospectively maintained, institutional review board-approved database of referred women with MUS-related symptoms who underwent MUS sub-urethral removal surgery was reviewed for several possible risk factors for VME including obesity, diabetes, menopause, HRT, and smoking. For this case-control study, we excluded women without the above defined demographic characteristics. Patients underwent MUS placement at outside facilities prior to presentation with MUS complication symptoms at our clinic. All MUS removal procedures were indicated based on presenting symptoms and conducted at our tertiary care center by the same surgeon. No external funding was utilized to conduct this study. A core outcome set was not used in the study's design, nor were patients involved in the design or conduction of this study. At the time of writing of this manuscript, a core outcome set for female pelvic floor disorders is ongoing (COMET Registration #981).
Demographic data were collected from participants' electronic medical record (EPIC, Verona, Wisconsin) by a third party not involved in the care of these patients and included age, body mass index (BMI), menopause, diabetes (none, insulin-dependent, type 2 diabetes mellitus), HRT status (none, vaginal, oral), smoking status (active, former, or never smoker), the date and location of MUS placement when the original operative report was available, and the date of SSR. Obesity was defined as BMI > 30 kg/m2. For former smokers, total pack-years and the date of quitting was also extracted from EPIC.
The location and size of VME were defined based on the IUGA/ICS classification of complications of mesh implants [9]. VME size (exposure defined as < 1 cm and extrusion as ≥ 1 cm) and location were confirmed based on intraoperative images that are taken systematically and are uploaded onto EPIC.
Patient records in EPIC were also reviewed for patient symptoms at presentation, including voiding dysfunction, vaginal pain, dyspareunia, recurrent urinary tract infections (RUTIs), and urinary incontinence [18]. Voiding dysfunction was defined as straining to void, sensation of incomplete emptying, urinary hesitancy, or decreased urinary stream. RUTIs were defined as ≥ 2 symptomatic infections within 6 months or ≥ 3 within 12 months, along with a positive urine culture [19]. Urinary incontinence symptoms were further classified as related to stress (SUI), urgency (UUI), or a mixture of both (MUI). All symptomatic VME patients in this series had failed prior local vaginal hormone cream treatment and therefore were considered for definitive excision of the exposed MUS. Because all patients with VME required sub-urethral MUS removal, patients having SSR for non-VME indications served as the control group.
Descriptive statistics were provided as medians and interquartile ranges for continuous variables and as frequencies and percentages for categorical variables. To assess the generalizability of our population, an initial comparison was made between smoking status by age in our cohort and the expected smoking distribution based on the age-matched proportions data of U.S. women in 2012 as previously reported [20]. These proportions were multiplied by the frequency of women by age group in our data and then summed for a total expected frequency. The differences in smoking status between the observed and the expected distributions were analyzed using the Chi-square test for specified proportions. Next, a Chi-square test for independence was used to test for an association between patients who underwent SSR surgery for VME or non-VME versus categorical patient characteristics, including smoking and diabetes status at time of surgery. For the same patient characteristics in patients with VME, the Fisher's Exact test was used to test for an association with size of exposure. The Kruskal-Wallis test was used to analyze for differences in continuous patient characteristics, including age at MUS removal and months of follow-up, by MUS removal indications (VME versus non-VME). Univariate and multivariate logistic regression was performed on smoking status and all characteristics meeting a 0.20 significance cutoff. Odds ratios were chosen as a method of comparison due to the retrospective nature of the study. All tests were performed at the 0.05 significance level using SAS 9.4 (SAS Institute Inc., Cary NC).
From 2005-2018, 496/499 (99%) women met study criteria. Three women were excluded due to undefined smoking status. Based on the proportions of smoking status by age group of U.S. women in 2012 [20] compared to the age distribution of our patients who underwent SSR, we did not observe significant differences between the expected and observed number of patients of each smoking status (p = 0.35) in our cohort. When looking at those with VME only, we again found no significant difference (p = 0.16) between the expected and observed number of patients by smoking status (Table 1).
Table 1: Expected number of smokers based on age distribution of patients with reference to U.S. 2012 report. View Table 1
Demographic information, the type of MUS removed, and patient symptoms at presentation among those with VME vs. non-VME are presented in Table 2. Although 5 patients had a history of cancer, including cervical, uterine, and breast, none were excluded due to an absence of immunosuppression, chemotherapy, or radiation to the pelvic area in these patients. Overall, 41/496 (8%) experienced VME. Comparing demographic factors between the two groups, women with VME had a shorter duration of follow-up (median 12 vs. 19 months; p = 0.0076), were younger (median age 54 vs. 58 years; p = 0.0070) and were less likely than those with non-VME to have voiding dysfunction (44% vs. 70%; p = 0.0005) and UTIs (41% vs. 59%; p = 0.033) at presentation. There was no association between VME and obesity, diabetes diagnosis, menopausal status, HRT, or smoking status. In the patients with VME, we were able to retrieve pack-year data (packs per day multiplied by years of smoking) for 17/20 of the current and former smokers. These patients had a median of 30 pack-years (IQR 10-40), and of the 15 former smokers with VME, the median years between quitting and sling placement was 5 (IQR 0-8). Two of the 15 former smokers quit after sling placement.
Table 2: Patient characteristics by exposure status. View Table 2
Location of exposure was noted on the left (51%), right (29%), at the midline (15%), and bilaterally (5%). Vaginal mesh extrusion was noted in (56%) of those with VME (examples provided in Figure 1A, Figure 1B and Figure 1C). A comparison of demographic characteristics between those with exposure and those with extrusion (Table 3) revealed that those with extrusion were more likely to have pain at presentation than those with exposure (74% vs. 39%; p = 0.031).
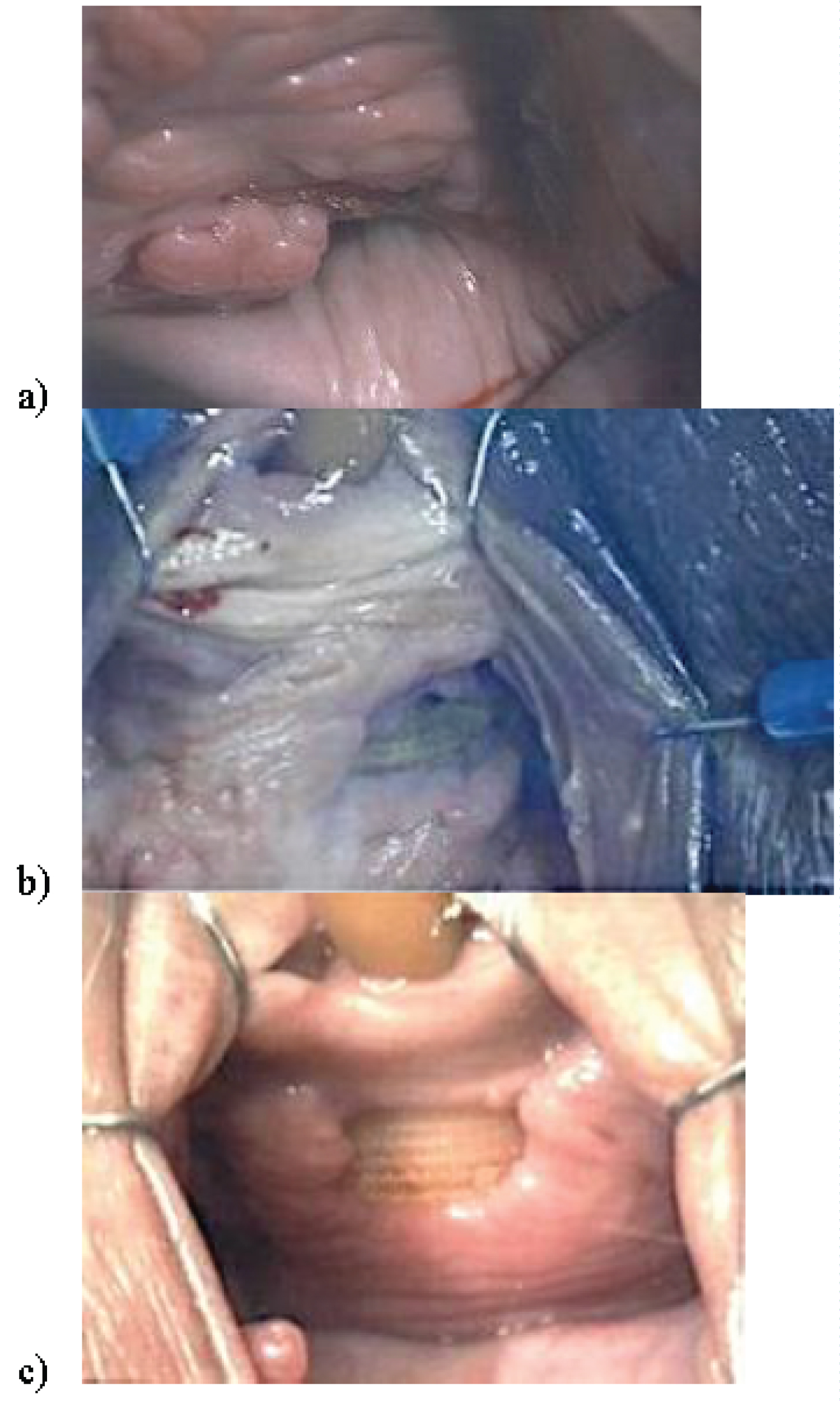 Figure 1: Intraoperative images of a) Vaginal mesh sling exposure b) Extrusion observed on the left, and c) Extrusion observed at the center.
View Figure 1
Figure 1: Intraoperative images of a) Vaginal mesh sling exposure b) Extrusion observed on the left, and c) Extrusion observed at the center.
View Figure 1
Table 3: Patient characteristics by size of exposure. View Table 3
Univariate analysis indicated that younger patients (OR 0.84; p = 0.015) and those with voiding dysfunction (OR 0.33; p = 0.0009) and RUTIs (OR 0.50; p = 0.036) were less likely to have VME. A negative association between age (OR 0.85; p = 0.043) and voiding dysfunction (OR 0.32; p = 0.0008) and VME persisted upon multivariate analysis (Table 4).
Table 4: Logistic regression of VME status in regards to patient characteristics. View Table 4
In this retrospective study of 496 women who suffered MUS-related complications, we found neither association in our cohort between obesity, diabetes, menopause, HRT, smoking, and the presence of VME, nor between MUS placement location and the presence of VME. We also found that women with VME were more likely to have a shorter duration of follow-up and to be younger compared to those in the non-VME group also undergoing SSR procedure. In addition, those with extrusion compared to exposure were more likely to have pain at presentation. Multivariate analysis indicated that older patients and those with voiding dysfunction were significantly less likely to have VME.
Strengths of our study include the size of the study population and all MUS removal procedures performed by a single surgeon with intraoperative photographs documenting VME size and location. This allowed classification of VME location and size according to the IUGA/ICS guidelines. Additionally, using EPIC, we were able to collate and compare risk factors between two groups of age-comparable women who underwent SSR procedure for either VME or non-VME indications. Our study was limited by its retrospective nature since all patients were referred to us for MUS-related complication symptoms, and all SSR-removal procedures to address their symptomatology were performed at a single institution with a study population consisting mostly of Caucasian women.
It is often stated that risk factors for poor tissue healing may lead to VME. Frank wound dehiscence may result from improper surgical closure or possibly from post-operative patient activities creating excess intraabdominal pressure. Since vaginal surgery is a clean-contaminated procedure, bacterial colonization of mesh implants occurs and may result in a localized infection. Such infection could cause inflammation, which weakens the surrounding tissues and increases the risk of VME [21]. In addition, hematoma formation [22] and impaired angiogenesis [23] following MUS insertion can impair healing of the vaginal wall and may precipitate VME.
In this study we analyzed several potential risk factors for VME. Smoking has been considered as a risk factor for VME because of decreased post-incision collagen deposition necessary for wound repair, leading to poor tissue health and impaired healing [24]. Conversely, tobacco use has also been associated with less oxidation and degradation of polypropylene hernia mesh, indicating a potentially protective role of smoking on mesh durability [25]. A review of the literature revealed only three manuscripts discussing smoking as a risk factor for VME after MUS [5,6,16].
Our study did not identify smoking as a risk factor for VME. Similarly, Linder and colleagues found no association between smoking and VME among 144 women after MUS placement over a median follow-up of 51 (0-137) months [16]. A larger study of VME among 1544 women three years after MUS placement by Cadish and colleagues also found no association between smoking and VME [6]. Conversely, Kokanali and colleagues reported smoking to be associated with VME in a study of 1439 women after transvaginal or transobturator tape placement with a mean follow-up period of 24 months [5]. However, none of these studies included a comparison cohort on the smoking status of women who underwent mesh removal for non-VME indications.
Decreased wound healing ability has also been observed in diabetes mellitus and obesity due to persistent inflammation, impaired angiogenesis, and reduced fibroblast activity [11,12]. Both Cadish and colleagues and Linder and colleagues found no association between diabetes and VME [6,16] whereas higher rates among diabetics were noted by Chen and colleagues and Kokanali and colleagues [5,26]. No association was found between BMI and VME in these studies [5,6,16,26]. In our cohort, neither diabetes nor obesity were associated with VME.
Another proposed mechanism for VME is an age-related decrease in estrogen production causing vaginal atrophy and increasing the risk of vaginal dehiscence and secondary mesh exposure [27,28]. Previous studies analyzing the correlation of age to VME, however, have yielded conflicting results [5,6,16]. Cadish and colleagues reported no significant difference in age, menopausal status, or systemic HRT use among women with and without mesh sling exposure after MUS placement [6]. Linder and colleagues reported age < 50 years, premenopausal status, HRT among menopausal women to be associated with vaginal mesh exposure following MUS compared to those without exposure following MUS placement [16]. Conversely, Kokanali and colleagues reported that those with VME were significantly older but no more likely to be postmenopausal than those without mesh exposure [5]. We observed that younger women were more likely to have VME. This observation may be due to the bother from VME compared to the degree of bother from symptoms such as voiding dysfunction and UTIs that prompted SSR among the non-VME cohort. Furthermore, in this study there was no significant difference between the two cohorts with respect to HRT, even when considering menopause.
MUS location has also been considered as a potential cause of MUS removal. A very large study by Gurol-Urganci and colleagues retrospectively reviewed outcomes of 95,057 women with a median age 51 (IQR 44-61) years who underwent MUS placement for SUI. They found that women who underwent retropubic sling placement were more likely to undergo subsequent removal compared to those with transobturator placement (3.6% vs. 2.7%; p < 0.001) [29]. In the Trial of Midurethral Slings, 597 women with a mean age 52.9 ± 11 years were randomized to receive either transobturator or retropubic mesh slings for SUI [30]. After 12 months follow-up, mesh sling exposure had occurred in 1 (0.3%) participant with transobturator and in 8 (2.7%) with retropubic slings (p = 0.30) [31]. After 24 months follow-up, mesh sling exposure occurrences increased to 9 (3.0%) and 6 (2.0%), respectively (p = 0.45) [8]. Our series supports these findings, as we did not find any difference in rates of VME based on MUS placement location or type of MUS.
In our study comparing symptoms at presentation among those with VME, we observed that those with extrusion were more likely to have pain at presentation compared to those with exposure. This finding is reasonable, as a larger area of VME represents a greater amount of tissue damage, which would likely cause more pain for the patient. Additionally, on multivariate analysis we found that those with voiding dysfunction were significantly less likely to have VME. Since obstructive voiding tends to occur with a MUS deep in the urethral wall, it is unlikely to be found vaginally exposed. Furthermore, women with obstruction or other non-VME indications for SSR may require additional management of their symptoms post-MUS placement, which may explain the longer duration of follow-up observed among those without VME in this study.
Despite being a well-established complication of MUS placement, overall VME rates documented in larger series have been low [5,6,8,31]. Cadish and colleagues reported VME to occur in 37/1544 (2%) women after 3 years [6], and Kokanali and colleagues reported a similar rate of 61/1439 (4%) over a mean follow-up of 24 months [5]. Over a longer follow-up duration (median 5.5 years), Gurol-Urganci and colleagues found the rates of mesh sling removal to be 1.4%, 2.7% and 3.3% at 1, 5 and 9 years, respectively [29]. This study, however, did not analyze whether mesh slings were removed due to mesh exposure or for another reason.
In this single tertiary care institution study managing referrals for MUS complications, we found no association between women who underwent MUS excision for VME versus non-VME indications for menopause, diabetes, hormonal replacement therapy, or smoking status, which persisted upon multivariate analysis. We also observed no association between MUS location and MUS excision for VME vs. non-VME indications. At present, it is difficult to predict which patients will experience VME following MUS placement, and further research is needed to elucidate potential risk factors that should be acknowledged when considering MUS placement for stress urinary incontinence.
None.
The authors have no conflicts of interest to declare.
No funding was received.
JAC: Data collection or management, Manuscript writing/editing; CF: Data collection, Manuscript editing; DR: Data collection, Manuscript editing, HA: Data collection, Manuscript editing, ALC: Data analysis, PEZ: Protocol/project development, Manuscript writing/editing. All authors reviewed the results and approved the final version of the manuscript.