The ABO antigens are expressed on the surface of red blood cells, epithelial cells, and endothelial cells. The association between different ABO blood group and several conditions such as VTE, CAD, and several neoplasms is well documented in the literature. The aim of this study is to examine the impact of ABO blood group on the biochemical failure after radical prostatectomy.
After Institutional research board approval; we retrospectively reviewed the radical prostatectomy database (1998-2012) to examine the association between the ABO blood group and biochemical recurrence. Hazard ratio (HRs) and their 95% confidence intervals were calculated using Univariable and multivariable Cox regression models.
385 patients underwent open radical retropubic prostatectomy between 1998 and 2012. Follow-up more than 2 months was available for 229 patients. Seventy-Five patients developed biochemical failure, defined as PSA > 0.2 ng/dl. The 10-year Biochemical recurrence-free survival were 53.6% (95% CI, 39.8%-65.7%) for blood group O, 58.0% for blood group A (95% CI, 43.1%-70.3%) and 61.8% (95% CI, 33.1%-81.1%) for blood group B. The log-rank test showed no significant associations between blood group and PSA failure at the univariate level and Multivariate. On the other hand, pre-op PSA, Gleason score, surgical margin and extra-prostatic extension were significantly associated with biochemical recurrence-free survival.
Radical prostatectomy, Biochemical recurrence, ABO blood group, Prostate cancer
One-third of patients who undergo radical prostatectomy will develop biochemical recurrence as a herald sign of disease recurrence [1]. Intriguingly, the outcome of those patients varies; some will have a local recurrence while other will progress to metastatic disease. There are multiple clinical and pathological features that predict the biochemical recurrence such as Gleason Score, Lymphovascular invasion, Seminal vesicle involvement and positive surgical margins [2]. Recently, there has been heightened interest in exploring the molecular basis of this heterogeneity in the outcome of patients with biochemical recurrence. Ross, et al. found that genomic classifier "decipher" has better ability to predict progression after biochemical recurrence than nomograms based on clinic-pathological features alone [3]. Moreover, other factors such as the use of metformin, statins, and smoking may alter the risk of biochemical recurrence.
The ABO antigens are expressed on the surface of red blood cells, epithelial cells, and endothelial cells [4]. Their immunological function is well-studied, and they appear to play a role in the pathogenesis of diseases. The association between different ABO blood groups and multiple conditions such as venous thromboembolisms (VTE), coronary artery disease (CAD), and several neoplasms has been well documented [5,6]. As in pancreatic cancer patients with blood type A as compared to blood type O had a greater risk of developing pancreatic cancer, however, patients with blood type B or AB did not [6].
The association between blood group and prostate cancer isn't well studied.
Due to the long nature of the disease, we opted to study the impact of ABO blood group on soft clinical sign namely biochemical recurrence after radical prostatectomy.
After Institutional review board approval, we retrospectively reviewed the radical prostatectomy database at the American University of Beirut between (1998-2012) and studied the association between the ABO blood group of 385 patients and subsequent biochemical recurrence. Follow-up more than 2 months was available for 229 patients. Seventy-Five patients developed biochemical failure, defined as PSA > 0.2 ng/dl in two consecutive measurements. ABO status, pre-op PSA, Gleason score, pathological stage, the status of surgical margins of the RP specimen were recorded and reviewed. Tumors were staged according to the 2009 TNM classification.
All statistical analyses were conducted using SPSS version 23.0 and STATA version 13.1 for Windows. All tests were two-sided and a p-value < 0.05 was considered significant. The following variables were considered for statistical analysis: age at RP, preoperative PSA level, ABO blood group, Gleason score, extraprostatic extension, seminal vesicle involvement, positive surgical margin and adjuvant therapy which was included as a time-dependent covariate. Continuous variables were summarized using means and standard deviations (SD) and categorical factors using frequencies and percentages. Variables were compared across blood group categories using the chi-square test, Fisher's exact test, independent samples t-test and one-way ANOVA as appropriate. Kaplan-Meier curves were plotted and compared across the categories of the main independent variable, blood group, using the log-rank test. Univariable and multivariable cox regression models were carried out to determine independent predictors of PSA failure. Variables that was significant at p < 0.2 ng/dl at the univariable level was entered into the multivariable model. Age of patients and blood group were forced into the multivariable model. Hazard ratios (HR) and their 95% confidence intervals (CI) were reported. The proportional hazards assumption was tested with Schoenfeld residuals. The ability of the cox model to discriminate between PSA failures and non-failures was measured by the Harrell's c statistic.
Data from 229 patients with prostate cancer were retrieved for this study. There were only 14 patients with the blood group AB and were therefore removed from the analysis for model convergence. The final study sample included 215 patients with mean age 62 years (SD = 6.10). Ninety patients (41.9%) had blood group O, 85 patients (39.5%) had blood group A and 40 patients (18.6%) had blood group B. Seventy-one patients (33.0%) had a Gleason score < = 6, 112 (52.1%) had a Gleason score equal to 7 and 32 patients (14.9%) had a Gleason score > = 8. Sixty patients (27.9%) had an extraprostatic extension, 108 patients (50.2%) had a positive surgical margin and 26 patients only (12.1%) had a seminal vesicle involvement. As for the pathological stage, 48 patients (22.3%) had T2a and T2b disease, 103 (47.9%) had T2c and 64 (29.8%) had T3. The mean pre-surgery PSA score was 8.89 (SD = 6.65). Characteristics of the total sample are presented in Table 1. We also compared the distributions of the clinical characteristics across the blood groups and found no significant associations (Table 1).
Table 1: Clinical characteristics of the total sample and across ABO blood group. View Table 1
Of the 215 patients, PSA failure was observed in 75 (34.9%) patients. Median follow-up time was 51 months (Interquartile range = 19-104). 10-year survival curves of each blood group are shown in Figure 1. The 10-year survival rates were 53.6% (95% CI, 39.8%-65.7%) for blood group O, 58.0% for blood group A (95% CI, 43.1%-70.3%) and 61.8% (95% CI, 33.1%-81.1%) for blood group B. The log-rank test showed no significant associations between blood group and PSA failure at the univariate level (Table 2). Cox regression was carried out to determine the variables associated with PSA failure. At the univariate level, patients with a Gleason score 7 (HR = 2.00, 95% CI: 1.09-3.60) and patients with Gleason score greater than or equal to 8 (HR = 2.40, 95% CI: 1.16-4.97) were more likely to have failure compared to patients with a Gleason score less than or equal to 6. Patients with higher pre-surgery PSA were also more likely to have a failure (HR = 1.03, 95% CI: 1.00-1.07) as shown in Table 2. Due to the high association between extraprostatic extension and surgical margin, we created a new variable that takes the value of 1 if either extraprostatic extension or surgical margin is present and 0 if none. The combined variable was included in the regression. Patients with either extraprostatic extension or surgical margin were also more likely to have a failure (HR = 2.55, 95% CI: 1.51-4.31). Although blood group was not associated with failure at the univariate level, we included it in the multivariable cox regression model. In the multivariable model and after adjusting for the clinical characteristics of the patients, blood group remained not associated with PSA failure, while having either an extraprostatic extension or a surgical margin was associated with failure (HR = 2.57, 95% CI: 1.37-4.82, Harrell's c-statistic = 0.64) Table 3. Of the 215 patients, PSA failure was observed in 75 (34.9%) patients. Median follow-up time was 51 months (Interquartile range = 19-104). 10-year survival curves of each blood group are shown in Figure 1. The 10-year survival rates were 53.6% (95% CI, 39.8%-65.7%) for blood group O, 58.0% for blood group A (95% CI, 43.1%-70.3%) and 61.8% (95% CI, 33.1%-81.1%) for blood group B. The log-rank test showed no significant associations between blood group and PSA failure at the univariate level (Table 2). Cox regression was carried out to determine the variables associated with PSA failure. At the univariate level, patients with a Gleason score 7 (HR = 2.00, 95% CI: 1.09-3.60) and patients with Gleason score greater than or equal to 8 (HR = 2.40, 95% CI: 1.16-4.97) were more likely to have failure compared to patients with a Gleason score less than or equal to 6. Patients with higher pre-surgery PSA were also more likely to have a failure (HR = 1.03, 95% CI: 1.00-1.07) as shown in Table 2. Due to the high association between extraprostatic extension and surgical margin, we created a new variable that takes the value of 1 if either extraprostatic extension or surgical margin is present and 0 if none. The combined variable was included in the regression. Patients with either extraprostatic extension or surgical margin were also more likely to have a failure (HR = 2.55, 95% CI: 1.51-4.31). Although blood group was not associated with failure at the univariate level, we included it in the multivariable cox regression model. In the multivariable model and after adjusting for the clinical characteristics of the patients, blood group remained not associated with PSA failure, while having either an extraprostatic extension or a surgical margin was associated with failure (HR = 2.57, 95% CI: 1.37-4.82, Harrell's c-statistic = 0.64) Table 3.
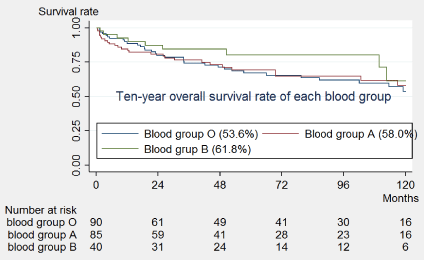 Figure 1: Overall survival curves by blood group.
View Figure 1
Figure 1: Overall survival curves by blood group.
View Figure 1
Table 2: Unadjusted associations between patient characteristics and PSA failure. View Table 2
Table 3: Multivariate Cox proportional hazard models of PSA failure. View Table 3
We studied ABO blood type as a prognostic biomarker in a patient with localized prostate cancer who underwent radical prostatectomy. Blood type was not associated with 10 years BCR free survival rate on univariate and multivariate regression model.
Interestingly, ABO gene is located on the long arm of chromosome 9 which also contains the coding genes for PCA3/DD3 and ABL oncogene [7,8]. In an elegant dissection of the genetic pathways involved in prostate carcinogenesis Ribeiro, et al. found that gain at chromosome 9q34 was found in cases with high Gleason score and associated with locally advanced disease and metastasis. However, the regression analysis failed to prove that gain at chromosome 9 is significant independent variable for local invasiveness and metastasis potential [9].
Recently, Ohno, et al. reported on the correlation between ABO blood group and BCR after RP; in contradiction to our finding, he found that O blood group was associated with longer BCR free survival in patients with negative surgical margin [10].
Increasing evidence indicates that Globo H and Lewis Y are highly overexpressed in various types of malignant tumors including breast, liver, prostate, and pancreatic cancer [11]. The H antigen is a precursor for the ABO blood group antigens located in intestinal mucosa and body fluids. Individuals homozygous for fut2 nonfunctional alleles would be considered non-secretors as they would not present their blood group antigens on their epithelial cells or secretions. 20% of people studied in various populations are considered non-secretors [12]. The penetration of fut2 gene in different populations varies and this may explain the inconsistency between our result and the previously reported results from other populations.
An association between Le(y) and SLe(x) antigens which are blood group related antigens was found to be upregulated in prostate cancer. Le(y) can be found in ducts and basal layer of the glandular epithelium in benign tissue however in carcinoma its expression is greatly increased. The SLe(x) antigen can be found only in ducts but not in the glandular epithelium of benign tissue while in carcinoma SLe(x) can rarely be found and in some cells found in focal to patchy distribution [13]. Moreover, a study by Kvist, et al. in 1992 found no association between prostate cancer and blood group antigens [14].
The inconsistency between the results of our study and the above studies can be attributed to several factors; for example, in Ohno, et al. study, there was a subgroup of patients with lymph nodes involvement, whereas all patients had localized disease in this study. Also, we can't not to ruminate the variation in geographic and ethnic distribution the ABO blood group allele.
It has been postulated that the ABO blood group antigen may affect the carcinogenesis by altering the levels of tumor necrosis factor-a [15]. Thus, it hampers cell adhesion and immune surveillance. Moreover, it has been documented that the loss of expression of blood group ABH is associated with worse prognosis of the bladder, colon, and head and neck cancers [16-18]. Indeed, these findings are engrossing in the light of our finding that there is no association between ABO blood group and BCR; which is a herald sign of distant metastasis.
There are multiple limitations to our study, first the retrospective nature of the study in addition to small sample size. There is an inherent loss of follow-up in our cohort attributed to the nature of referral from other countries. Moreover, we were unable to investigate our patients for their status as secretors/non-secretors and account for that variation in our study.
We can't preclude the role of ABO blood group on the disease biology and aggressiveness. The ABO blood group might be used to stratify the patients in future studies of personalized adjuvant treatment after surgery. Further studies are needed to elucidate this correlation.
ABO blood type as a prognostic biomarker was not associated with 10 years BCR free survival rate on univariate and multivariate regression model in patients who underwent radical prostatectomy for localized prostate cancer.