To evaluate serum copeptin levels in patients with obstructive sleep apnea (OSA) and healthy controls.
Fifty-eight newly diagnosed OSA patients and thirty-five healthy individuals were enrolled. OSA patients were divided into mild (5 ≥ AHI < 15), moderate (15 ≥ AHI < 30), and severe (AHI ≥ 30) groups. Copeptin levels were evaluated and compared between patients and healthy individuals. For the subgroup analysis, OSA patients were divided into two groups, AHI < 30 (mild-moderate) and ≥ 30 (severe) and copeptin levels were compared between the groups.
Copeptin levels were significantly higher in the OSA group compared to the healthy individuals (p = 0.001). In severe OSA patients (AHI ≥ 30), copeptin levels were significantly higher than in the mild-moderate group (p = 0.038). The analysis showed that copeptin levels > 4.41 had a 89% sensitivity and 71% specificity for APE (AUC 0.831, 95% CI 0.740-0.922).
Serum copeptin levels were found to be associated with OSA, severe OSA. Copeptin levels may be useful for monitoring and determining high-risk patients OSA patients.
Obstructive sleep apnea (OSA) is an important public health concern with numerous consequences at the societal level, including motor vehicle accidents, increased cardiovascular morbidity, metabolic dysfunction, and behavioral and cognitive deficit [1].
OSA is characterized by a decrease in, or complete cessation of, airflow despite ongoing efforts to breathe, and is related to oxygen desaturation and sleep fragmentation [2]. The prevalence of OSA has been estimated at 14% in men and 5% in women [3]. Diagnosis of OSA is usually made when a patient has an apnea-hypopnea index (AHI) ≥ 5, along with excessive daytime sleepiness [4].
Diagnosis of OSA usually requires overnight polysomnography (PSG) [1]. Overnight PSG is a significant undertaking and is relatively inaccessible to patients; waiting times for evaluations leading to diagnosis are commonly 3-6 months in the United States and worldwide [5]. Therefore, researchers have investigated more straightforward and less costly tests of potential diagnostic biomarkers for OSA. Several studies have shown that patients with OSA have elevated levels of proinflammatory cytokines, cellular adhesion molecules, and activated circulating neutrophils [6,7]. Interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and high-sensitivity C-reactive protein are the most frequently evaluated biomarkers in OSA [1].
Copeptin is a novel biomarker that has recently been discussed in terms of its role in OSA. Copeptin is the C-terminal fragment of pre-provasopressin, which includes a 39-amino acid glycopeptide [8,9]. Copeptin is released with vasopressin and can be used instead of the latter agent [10-12]. It is an easily measurable peptide [8,13] and its levels may reflect hemodynamic and osmoregulatory disturbances [2]. Copeptin has been shown to be an important marker for eventual fatal diseases such as stroke, heart failure, and severe sepsis [14]. It is also a prognostic marker in patients with cardiovascular diseases [9,11,15]. There are a few, controversial, studies on the use of copeptin as a biomarker in patients with OSA. The aim of this prospective case-control study was to evaluate the serum copeptin levels in patients with OSA, as well as in healthy individuals.
This cross-sectional study was conducted in the Department of Chest Diseases and Department of Otorhinolaryngology, Head and Neck Surgery between August 2016 and July 2017. Approval was given by the local Ethics Committee and informed consent was obtained from all participants.
Fifty-eight newly diagnosed OSA patients and 35 healthy individuals were enrolled in this study. Diagnosis of OSA was made when a patient had an AHI ≥ 5 by PSG, as well as excessive daytime sleepiness. OSA patients were divided into mild (5 ≥ AHI < 15), moderate (15 ≥ AHI < 30), and severe (AHI ≥ 30) groups [16]. The control group were randomly selected from among healthy individuals who attended the chest diseases outpatient polyclinic for routine checks. They were evaluated using the Epworth Sleepiness Scale (ESS). Healthy individuals with an ESS score < 5 were included in the study. Patients younger than 18 years of age and pregnant women were excluded from the study. Copeptin levels were evaluated and compared between in two groups.
In subgroup analysis, OSA patients were divided into two groups according to AHI levels: < 30 [mild-moderate] or ≥ 30 [severe]. The copeptin levels were compared between the groups. Additionally, cardiovascular diseases such as hypertension and coronary artery disease were compared between the groups.
PSG recordings were performed using Philips Respironics equipment in the same center. Data were recorded by electroencephalography, electrooculography, and chin and bilateral anterior tibialis surface electromyography, to explore thoracic and abdominal movements. Oxygen saturation was measured with pulse oximetry. Oronasal air flow was measured by both oronasal thermal sensors and a nasal pressure cannula. Sleep state scoring and respiratory events were evaluated according to the American Academy of Sleep Medicine manual of sleep and associated events (ver. 2.4). AHI was calculated according to the mean number of apneas and hypopneas per hour of sleep [17].
Blood samples were collected from subjects after a minimum 8-hour fast. The collected samples were centrifuged at 1,600 g for 15 min and the serum was separated and stored at -80 ℃ until analysis. The serum copeptin levels were measured using an autoanalyzer (Eastbiopharm Co., Ltd, Hangzhou, China) with a commercial kit using an enzymatic colorimetric method.
IBM SPSS Statistics for Windows software (ver. 20.0; IBM Corp., Armonk, NY, USA) was used for the statistical analyses. Normal distribution of the data was evaluated with the Kolmogorov-Smirnov test. Variables were compared in terms of the mean and standard deviation. Student's t-test was used to determine differences between group means for continuous variables. The Mann-Whitney U test was used to assess non-normal data. The χ2 test was used to evaluate categorical variables, and p < 0.05 indicated significance.
This study enrolled 58 patients with OSA (mean age, 59.66 ± 19.29 years) and 35 healthy individuals (mean age, 54.53 ± 8.99 years). No significant difference was found between in two groups in terms of age or gender (both p > 0.05). The demographic data and laboratory findings of the OSA and healthy subjects are summarized in Table 1. Copeptin levels were significantly higher in the OSA group compared to the healthy subjects (p = 0.001) (Figure 1). In 38 patients with severe OSA (AHI ≥ 30), copeptin levels were 9.91 ± 7.10 ng/mL, while copeptin levels were 6.54 ± 3.45 ng/mL in 20 mild-moderate OSA patients (AHI < 30). In patients with severe OSA, copeptin levels were significantly higher than in the mild-moderate group (p = 0.038) (Figure 2). The analysis showed that copeptin levels > 4.41 had a 89% sensitivity and 71% specificity for APE (AUC 0.831, 95% CI 0.740-0.922) (Figure 3).
Table 1: Demographic data and laboratory parameters of the OSA and healthy subjects. View Table 1
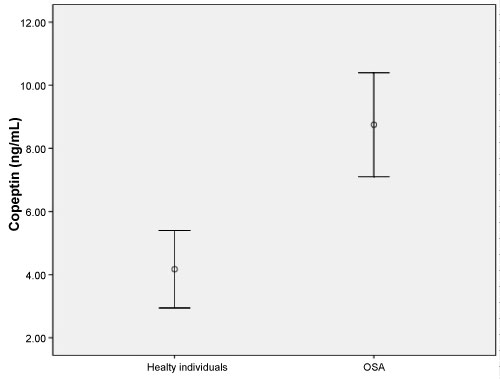 Figure 1: Copeptin levels (ng/mL) in the two groups (p = 0.001). View Figure 1
Figure 1: Copeptin levels (ng/mL) in the two groups (p = 0.001). View Figure 1
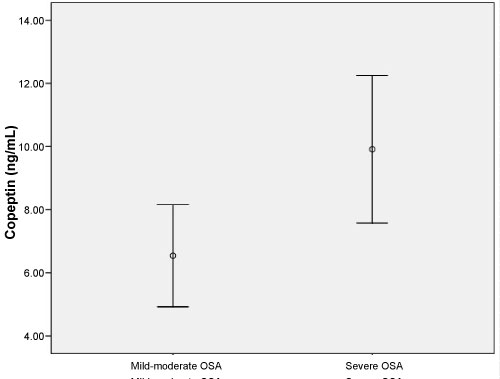 Figure 2: Copeptin levels (ng/mL) in mild-moderate OSA and severe OSA (p = 0.038). View Figure 2
Figure 2: Copeptin levels (ng/mL) in mild-moderate OSA and severe OSA (p = 0.038). View Figure 2
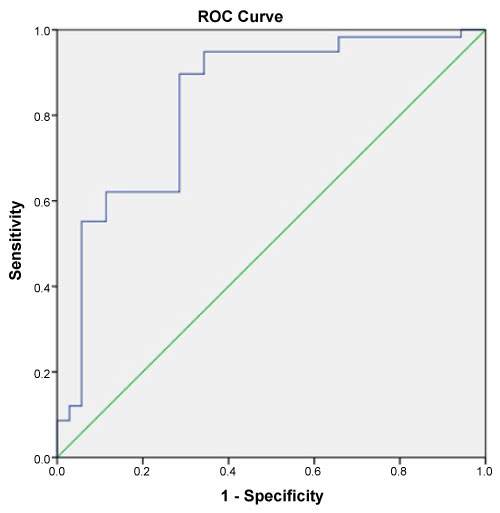 Figure 3: ROC curve for Copeptid associated with obstructive sleep apnea. View Figure 3
Figure 3: ROC curve for Copeptid associated with obstructive sleep apnea. View Figure 3
Comorbidities with OSA patients shows Table 2. There were not significant differences between the severe OSA and mild-moderate OSA in terms of cardiovascular diseases such as hypertension and coronary artery disease (respectively p = 0.106, p = 0.653).
Table 2: Comorbidities of the OSA patients. View Table 2
Copeptin levels in this study were found to be significantly higher in the OSA group compared to the healty individuals. Moreover, copeptin levels were significantly higher in patients with severe OSA compared to the mild and moderate groups. Thus, the copeptin levels appears to be associated with OSA severity.
OSA is characterized by recurrent airway obstruction of the posterior pharynx during sleep, leading to hypoxemia and arousals [16]. Intermittent hypoxia is associated with disruption in the normal autonomic and hemodynamic responses to sleep, which are associated with increased sympathetic activity [4,18,19]. The repetitive hypoxia and reoxygenation seen in OSA leads to oxidative stress and the generation of reactive oxygen species (ROS), which may play an important role in activating the inflammatory response among patients with OSA [4]. The relationship between OSA and proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, has been demonstrated in a number of studies [20,21]. It has been shown that decreased levels of adiponectin, a cytokine-like hormone, confers an anti-inflammatory effect in OSA [16,22,23].
Systemic oxidative stress in OSA is an important mechanism linked to cardiovascular disease and metabolic disturbances [24]. In patients with OSA, increased sympathetic activation and inflammatory mediator levels are associated with comorbidities such as hypertension, diabetes mellitus, heart disease, depression, and anxiety disorders [25]. Repeated sleep-related apneas have been associated with cyclic variations of sympathetic muscle activity, and each apneic event leads to increased sympathetic traffic [26]. Plasma and urine norepinephrine levels are increased in OSA patients [27]. The hypothalamic-pituitary-adrenal [HPA] axis plays an important role in regulating alertness and sleep, and likely also in energy balance. Hypoxemia, arousals, and sleep fragmentation alter the normal function of the HPA axis [4]. Activation of the HPA axis is caused by the presence of stressors and leads to an increase in the concentrations of adrenal stress hormones, such as corticotropin-releasing hormone and vasopressin [13,28]. Vasopressin plays a key role in cardiovascular homeostasis. It is produced in the hypothalamus and its release leads to altered plasma osmolality, stress, and hypotension [10]. However, it is unstable in plasma and has a short half-life [10-13]. Copeptin released with vasopressin can be used instead of vasopressin as a biomarker [10-12]. Copeptin is a biomarker related to the stress response [14]. Copeptin levels have been associated with sepsis, congestive heart failure, acute MI, and lower respiratory tract infections [11,29,30]. Recently, a few studies of the relationship between copeptin and OSA have been published [2,31-33]. Cinarka, et al. [2] showed that copeptin levels in patients with OSA were significantly higher than in the control group [2]. Moreover, they were also significantly higher in the severe OSA group (AHI ≥ 30) compared to those with an AHI < 30 in the present study. Akkoyun, et al. [31] found increased serum copeptin levels in OSA patients [31]. A further two studies noted that copeptin levels were not associated with OSA [32,33]. In the present study, we found that serum copeptin levels increased in OSA.
This study had certain limitations because it included a study population of limited size. Additionally, in this study was compared OSA patients and healthy individuals. Healthy individuals did not have any comorbidities. OSA group have comorbidities such as cardiovascular diseases that may affect copeptin levels. Therefore, OSA patients were divided into two groups as mild-moderate and severe. Cardiovascular diseases such as hypertension and coronary artery disease were compared between the groups. There were no significant differences between the groups.
As conclusion, copeptin has been increasingly linked with a number of illnesses. Although the use of copeptin as a biomarker for cardiac events is well established, the use of copeptin levels as a biomarker in OSA patients is controversial. In the present study, serum copeptin levels were associated with OSA. Copeptin may be associated with particularly severe OSA. Copeptin levels may also be useful for monitoring and determining high-risk patients OSA patients. Multicentric studies are needed in future.
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors and there is no conflict of interest.