During the last few years there has been an increase in consumption of psychostimulants, substances that augment brain activity, in Brazil and around the world. It’s noted that the utilization of these substances involves not only medical need, but also recreational purposes, being the latter identified as indication of substance abuse. Among these stimulants, there are anorexigenics, such as amphetamines and derivatives. Amphetamines are drugs that stimulate the central nervous system, capable of generating euphoric states, enhanced vigilance, endurance and anorexia. Adulteration is a very similar phenomenon within the drug market.For this, it is always a challenge for police intelligence to develop accurate, robust and especially cheap analytical techniques in order to unravel the pattern of drug tampering. Here we demonstrate a highly reliable, reproducible and low-cost method, namely the use of flame-ionized gas chromatography for detection and quantification of amphetamines. Standardization of our method was based on analysis of amphetamine, methamphetamine, amfepramone, methylenedioxymethamphetamine and fenproporex. Calibration curve linearity for each of the analytes was obtained by injecting five replicates of the following concentrations: 1000 µg.mL-1, 500 µg.mL-1, 250 µg.mL-1, 125 µg.mL-1, 75 µg.mL-1, 50 µg.mL-1, 25 µg.mL-1 and 10 µg.mL-1. Adherence to established parameters were followed throughout. The calibrations curves corresponding to amphetamine, MDMA and amfepramone presented a correction linear coefficient of (r2) = 0.9991906, (r2) = 0.9993966 and (r2) = 0.9996338 and another validation parameters also meet the requirements of regulatory bodies.
Validartion, Amphetamine, MDMA, Amfepramone
Amphetamines have as basic structure the β-phenylisopropylamine. Small chemical modifications of this molecule generate a large number of pharmacologically active compounds such as amphetamine, methamphetamine, methylenedioxymethamphetamine (MDMA), amfepramone (DEP), fenproporex (FEM), among others. In Brazil, in addition to the amphetaminic derivatives, the use of mazindol is very common, although it does not derive from the same chemical group; it has anorexigenic properties with very similar mechanism of action [1,2].
Ingestion of amphetamines enhances the central nervous system (CNS) activity and allows users to acquire higher levels of competence in order to perform activities for longer periods of time and with less fatigue. During the first few hours after ingestion, the user gets a sensation of well-being and disposition, but after these effects subside, they become irritable, depressed, and often overtaken by uncontrollable somnolence [3].
The use of these drugs, in a repeated and prolonged manner, leads to tolerance, that is, the decrease of their effect when ingested in recommended amounts. Consequently, the doses will be increased by the users with the intent to achieve the desired effect. In this way there is a high probability of causing dependence and a compulsive, or addictive behavior. In addition, these drugs have contraindications for use during pregnancy, or for patients with hypertension, cardiovascular problems, hyperthyroidism, or glaucoma, as well as individuals with a history of drug abuse.
According to data from the 2009 report from the National Controlled Products Management System (from Portuguese: Sistema Nacional de Gerenciamento de Produtos Controlados-SNGPC), Brazil was among the largest consumers of anorexigenic stimulants, including substances that are controlled by specific legislation [4]. Among those substances are amfepramone, fenproporex and mazindol. Dispensing of these substances occurred, almost entirely, in compounding pharmacies. Due to the characteristic effects of these drugs, which include low therapeutic index and high potential for abuse, there’s a need for stricter monitoring of quality control in dispensing establishments [5].
In addition to the medical use of these controlled substances, the use of illicit derivatives such as amphetamine, methamphetamine and MDMA (ecstasy), is popular among the youth who seek to experiment with drugs in order to trigger pleasant sensations. The effects of these drugs resemble those caused by cocaine, which might result in an orgasmic experience, most often violent and instantaneous, also known as rush, which is triggered during consumption, followed by physical and psychic excitement [6].
The use of these substances brings about increased mental acuity and improved mood. They also decrease fatigue and cause a sensation of increased energy and muscular strength, self-confidence and euphoria, followed by a feeling of tiredness and/or depression. Consequently, users eventually develop a depressive condition, which can progress to an irreversible psychotic state, comparable to paranoid schizophrenia [6].
Due to the above characteristics and the constant appearance of new amphetamine derivatives in the market, it is necessary to increase the rigidity of existing legislation, or even to create new regulations for strict control of the use of these substances. It is also important to adapt and enable laboratories for efficient analysis of large number of substances that have similar chemical structure using a method that is fast, accurate and economical. In addition, industrial laboratories are always looking for efficient, inexpensive and increasingly rapid methods for the analysis of their raw materials and finished products, especially in recent years due to increased production of pharmaceuticals in the country.
The present work was aimed at developing a method for the quantification of amphetamines by gas chromatography with a flame ionization detector, which is a simple, reproducible, reliable and importantly, an affordable technique.
Samples analysis was performed in the Legal Medical Institute of São Paulo, Brazil. Secondary standards were used for amphetamine, methamphetamine, fenproporex, amfepramone and mazindol. All substances used in this work were of high purity. The secondary standards were purchased by Sigma-Aldrich.
The analysis were conducted ina Shimadzu® Gas chromatographer, model GC2010, coupled with a flame ionizing detector (FID), split/splitless injector, 60 meters capillary column 100% metilpolisiloxano® ref. DB-1, internal diameter 0.25 mm and film thickness of 0.25 µm. The system was coupled to a computer containing acquisition and analysis software GC solution version 2.31.00, coupled to an automatic injector Combi Pal CTC Analytics AG. The injection volume was 1 uL and the gasses used were special for gas chromatography (CG): hydrogen, nitrogen White Martins, DIG - SP. The following chromatographic condition was used for the analyzes: Column with 99% polysylsiloxane DB1 (60 m × 0.32 mm × 5 μm); drag gas (hydrogen) at a flow rate of 5.0 mL.min-1; injector temperature was 280 °C; detector temperature was 300 °C; column temperature (programming) was 160 °C for 1 minute, 12.5 °C/min to 210 °C, 210 °C for 1 minute, and the total running time was 5.5 minutes. Data were processed using GC solution Version 2.31.00 software. To obtain the chromatograms, the raw data were extracted from the software of each equipment.
For the other processes it was used Ultrasonic bath Limp Sonic®; transferpette automatic pipettes, ependorff®, with different volumetric capacities; clear glass vials with 2.0 mL capacity, silicone septa and screw caps, Supelco®; glass beads, beckers, volumetric flasks, J.T.Baker® PA grade methanol, PA J.T.Baker® chloroform, PA J.T.Baker® ether.
Selectivity testing utilized methanol solutions of the following drugs: fenproporex, mazindol, amphetamine, methamphetamine and ecstasy. 20 mg of each of the drugs was measured and transferred into 10 ml round volumetric flasks, to which 9 ml of methanol was added. The volumetric flasks were bath-sonicated for 5 minutes, then homogenized and further diluted with the same solvent in order to reach working concentrations of 2000 ppm.
Standard curves and precision tests were performed by weighing 10 mg of amphetamine and methamphetamine. Those were transferred into 10 ml round volumetric flasks together with 5 ml of the stock solution of amfepramone at 2000 ppm, followed by volume completion to 10 ml, which brought the concentration of each solute to 1000 ppm. This initial concentration was used to prepare serial dilutions in order to obtain subsequent solutions of 705, 660, 570, 500, 340, 330, 285, 250, 125, 75, 50, 25, 20 and 10 ppm.
The ecstasy tablet dissolution procedure was performed in 5 ml of distilled water for plastic tubes with thread. To each tube was added 40 ml of extractive solvent composed of 1:1 ether:chloroform. All tubes were hermetically sealed and packed in the horizontal electric shaker and shaken vigorously for about 50 minutes. After stirring, they were filtered over anhydrous sodium sulfate and packed in a hood until complete evaporation of the solvent at room temperature. Resuspension of this adhered material was carried out on the walls and bottom of the becker, with the addition of 1.0 mL of methanol. This extract was used directly in the vitreous tubes for analysis in the chromatograph.
The method was validated, establishing the limits of detection (LOD), limit of quantification (LOQ), linearity, accuracy and precision intra and inter-day according to national and international guides.
Chromatographic parameters were determined according to protocol. For evaluation of specificity, we have used standard with known similar chemical structures, possible contaminants or compounds that might be present in manipulated formulas. These substances were: fenproporex, amphetamine, methamphetamine, ecstasy and mazindol. Table 1 shows median retention times of these drugs compared to amfepramone.
Table 1: Median retention times of tested drugs. View Table 1
Figure 1 shows a reference chromatogram after injection of solutions containing amphetamine, methamphetamine, amfepramone, MDMA, fenproporex and mazindol using conditions described in item 2. Notice that there’s no detection of mazindol in this chromatogram.
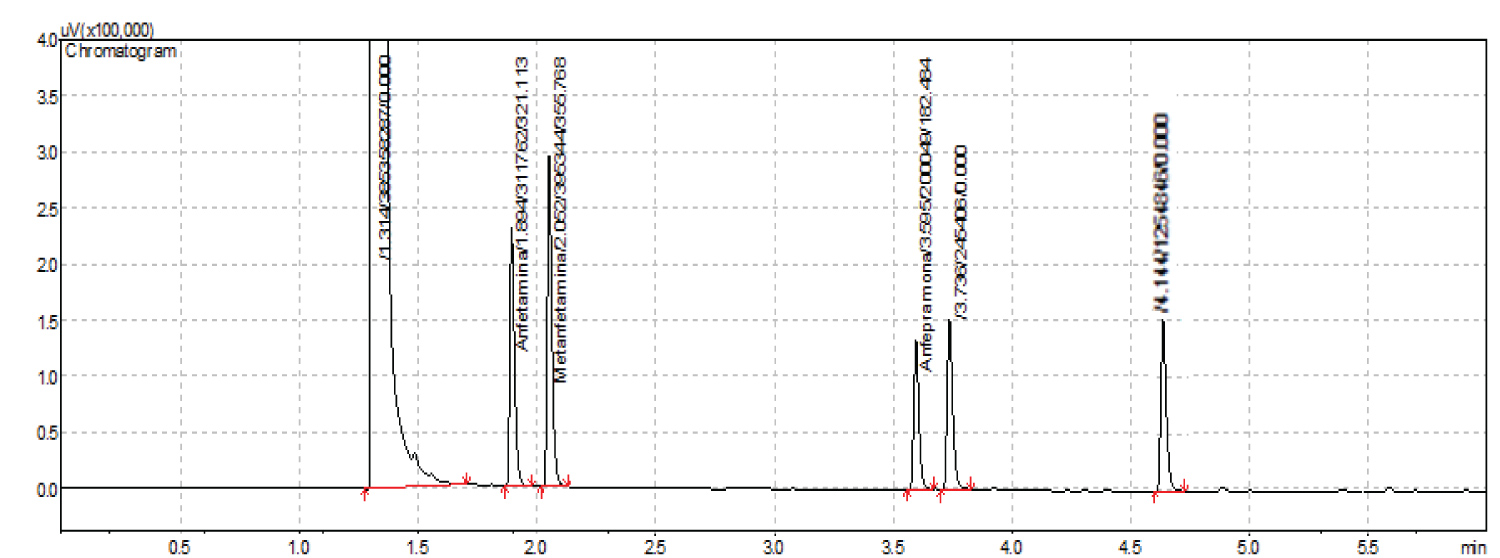 Figure 1: Chromatograph of solution containing fenproporex, amphetamine, methamphetamine, ecstasy and mazindol, in concentrations of 2000 µg.mL-1, according to established conditions.
View Figure 1
Figure 1: Chromatograph of solution containing fenproporex, amphetamine, methamphetamine, ecstasy and mazindol, in concentrations of 2000 µg.mL-1, according to established conditions.
View Figure 1
Calibration curves for determination of linearity were obtained by injections in multiples of five. Calibration curves were obtained for amphetamine, methamphetamine andamfepramone for the following concentrations: 1000 µg.mL-1, 500 µg.mL-1, 250 µg.mL-1, 125 µg.mL-1, 75 µg.mL-1, 50 µg.mL-1, 25 µg.mL-1 and 10 µg.mL-1. Each standard curve was normalized to null concentrations. Calibration curves for each tested drug are shown in Figure 2, Figure 3 and Figure 4, respectively.
 Figure 2: Amphetamine calibration curve.
View Figure 2
Figure 2: Amphetamine calibration curve.
View Figure 2
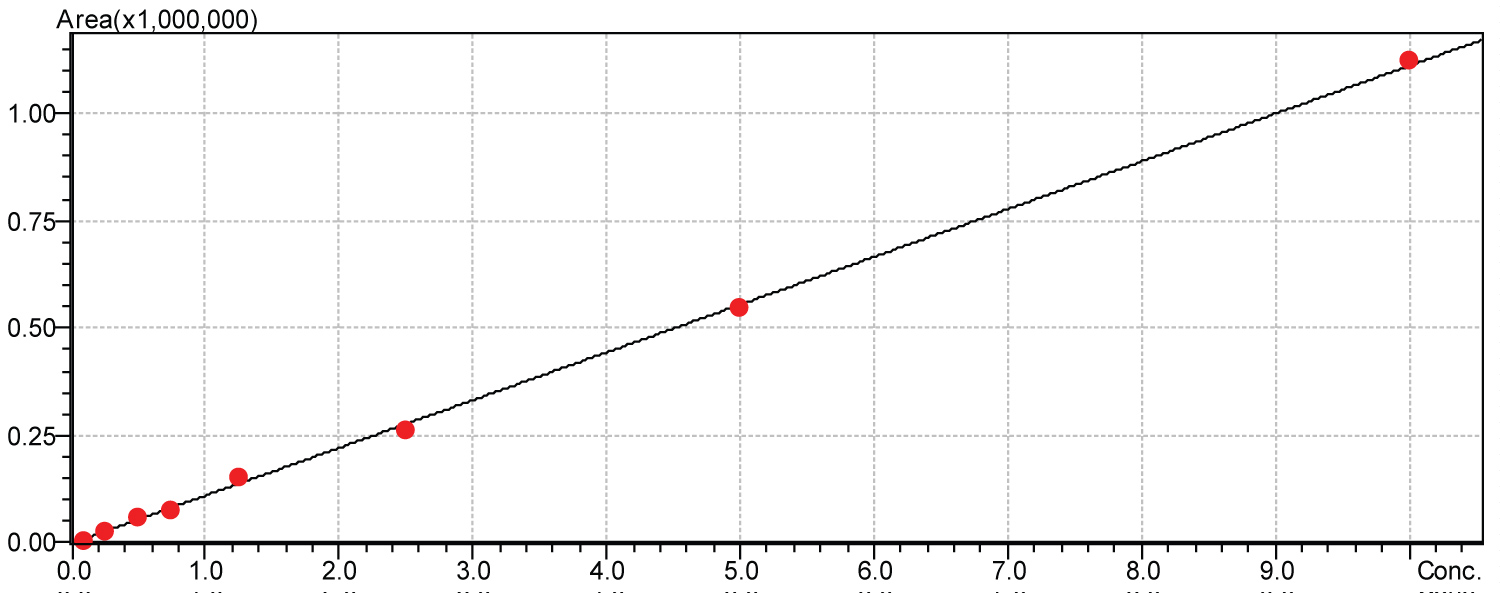 Figure 3: Methamphetamine calibration curve.
View Figure 3
Figure 3: Methamphetamine calibration curve.
View Figure 3
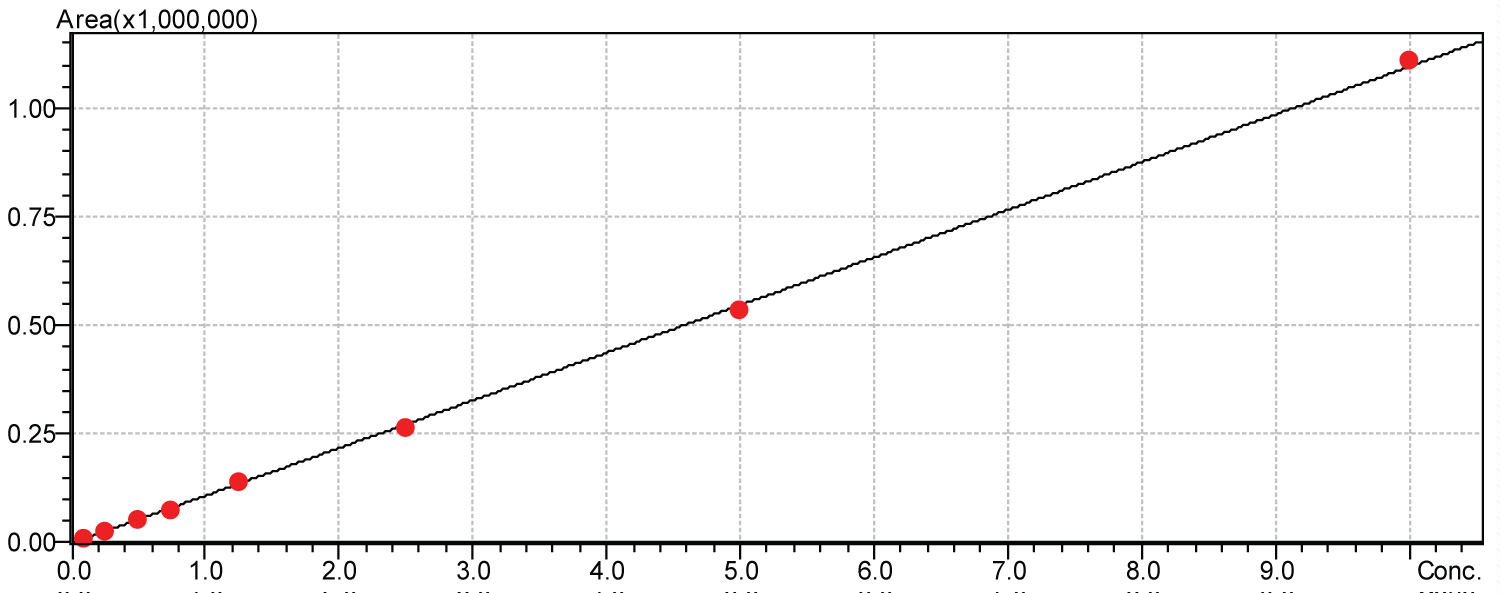 Figure 4: Amfepramone calibration curve.
View Figure 4
Figure 4: Amfepramone calibration curve.
View Figure 4
The calibration curve corresponding to amphetamine followed a linear equation with an angular coefficient of 97087.99 and a correction linear coefficient of (r2) = 0.9991906. Formula 1 represents the described equation: Y = 97087.99x.
Calibration curve obtained for amphetamine represented a linear equation with an angular coefficient of 111124.0 and a correction linear coefficient of (r2) = 0.9993966. Formula 2 represents the described equation: Y = 111124.0x Calibration curve obtained for amfepramone represented a linear equation with an angular coefficient of 109625.6 and a correction linear coefficient of (r2) = 0.9996338. Formula 3 represents the described equation: Y = 109625.6x.
Precision was determined utilizing multiples of 6 injections of each analyzed drug. Precision for linearity test was determined using 3 different concentrations: low, median and high. For amphetamine, the concentrations used were 20 µg.mL-1, 285 µg.mL-1 and 570 µg.mL-1. For methamphetamine, concentrations used were of 10 µg.mL-1, 340 µg.mL-1 and 705 µg.mL-1. For amfepramone, concentrations used were of 10 µg.mL-1, 330 µg.mL-1 and 660 µg.mL-1.
Determination of precision took place over 2 days. In the first day, samples were prepared and ran by a designated person and on the second day, standard samples were prepared and ran by a different person. Coefficients of variation are represented in Table 2.
Table 2: Variation coefficients for amphetamine, methamphetamine e amfepramone. View Table 2
We have taken into account that precision and accuracy should have been performed with certified samples and that simply alternating between researchers who are performing the experiments does not guarantee method precision. However, during the time we were executing the experiments in Brazil, a new mandate, order 344/98, was passed by the Office of Health Vigilance (Secretaria de Vigilanciaem Saude, SVS) which forbid purchasing of certified drugs considered as substances of abuse. Among the forbidden substances were included metabolites and degradation products of the same, which added an unexpected level of difficulty in carrying out experiments as originally planned. All solutions used for standardization of calibration curves were purchased and stocked prior to implementation of the new mandate. Therefore, it was not possible to perform experiments with the rigidity required by the literature and we had to limit calculations of precision based on intra-day and intra-researcher.
The present work focused on the development of a simple, fast and inexpensive analytical method capable of assisting in the identification and quantification of amfepramone. The concept of the development of this work is based on consistent increase in consumption of amphetamines and the constant attempt by authorities to implement strict control over these types of substances. Nowadays, the consumption of such substances has been practically abusive, mainly by the female population, which, manipulated by idealistic concepts of beauty imposed by society, end up succumbing to chemical shortcuts, such as the consumption of anorexigenic drugs to achieve weight reduction and peer-oriented goals [7]. Abusive consumption of amphetamines ends up diverting the true function of these drugs, which should be to aid in the treatment of obesity, with the concomitant implementation of optimized eating habits and the practice of regular physical exercise [8].
In addition to the increased prescription use of these drugs, their illicit useshows a similar growth rate. Popular uses of amphetamines include a wide range of reasons, for example increased wakefulness, as in the case of long-distance truck drivers; increased vigilance and productivity, as in the case of students who want to improve their academic performance; increased alertness and attentiveness to details, as in the case of professionals such as health care workers; or simply to bring about euphoric sensations by people who are looking for stimulants for social interactions [9].
Both prescription and illicit use of these drugs have serious side effects and often irreparable damage to health, which in itself would justify the need for strict governmental control and monitoring. However, in order to effectively monitor usage of these drugs, there’s a need for development of reliable tools for detection and analysis. Analysis tools are needed at several levels: in industry in order to guarantee the supply of effective, safe and high-quality medicines; in toxicology laboratories, for the analysis of substances seized as well as for determination of drug specificity and metabolites after consumption. Throughout these levels, both speed and economic viability are important requirements when choosing/developing methods of analysis. Such laboratories operate under strict timelines and demand methods of analysis that are practical, reliable, fast and that allows reproducibility. Therefore, implementation of methods such as gas chromatography, which possess these characteristics, would be well accepted for the above-mentioned purposes [10].
Once a choice of analytical method is made, it’s important that it gets validated. Here the method we have developed and described in section 3.5 has shown proof of validation and suitability. In specificity analysis, our chromatographic method has shown peaks that are distinct and resolved to a satisfactory degree for quantitative analysis, even in relation to peaks with near retention time, such as amphetamine and methamphetamine, or amphetamine and ecstasy. As can be verified, mazindol was not detected in the chromatographic assay. This may be related to the fact that its structural formula is not similar to the structure of amphetamines [11].
Linearity was performed as shown in the results. The calibration curves were normalized to zero, generating more rigor in obtaining the coefficient of linear correlation. The coefficients obtained for amphetamine, methamphetamine and amfepramone were all higher than 0.999. ANVISA stipulates the reliability of an analytical and linear method provided that the calibration curve obtained has r2 equal to or greater than 0.99, therefore, based on this criterium, the method developed has high linearity/reliability for the three drugs analyzed [12].
There was no possibility of conducting the linearity test for fenproporex because the standards for fenproporex were degraded, which would impair the analysis, and would not generate reliable results. This test also could not be performed for MDMA, by the absence of a standard, having available only tablet samples [13].
Tests for accuracy were performed as described in the results section. The two types of tests, intra-day and intraoperative, were performed simultaneously, generating in this manner a greater number of variables, thus enabling a more rigorous test. As can be seen, all coefficients of variation obtained were lower than the 5% limit recommended by ANVISA. These data contradict the findings of Franck (2008) [14] due to greater accuracy of the method and minimal coefficients of variation. Increased accuracy of our method may be due to the use of an automatic injector, which allows greater repeatability and reproducibility of injections, with minimization of variables Franck (2008) [14].
As previously stated, it was not possible to perform accuracy tests, since this test requires primary standards and the commercialization of standards of illicit substances present in ordinance 344/98 was prohibited when the legislation went into effect. Purchasing prohibition has halted a complete validation of the proposed method. Regardless of purchasing restrictions, the data obtained with our method is proven to be highly reliable and validates its implementation for use in toxicology, diagnostic and forensic laboratories, assisting in the identification of amphetamines mostly used today, and in the quantification of amphetamine, methamphetamine and amfepramone with an assay that takes only 6 minutes to run. Its use in industrial laboratories is also viable, which would guarantee great agility in the analysis of raw material content and finished product content, in addition to the fact that the method requires a small amount of sample and is relatively inexpensive when compared to other instrumental techniques [15].
The present work demonstrated the development and validation of a flame ionizing gas chromatography detection and quantification method for analysis of amphetamines. This method has parameters befitting the objectives of the work proposed. It presented specificity and linearity for analysis of amphetamines tested within the scope of this work. The amphetamines tested were: Amfepramone, fenproporex, amphetamine, methamphetamine and ecstasy, which combined detection was shown to be feasible. The method was shown to be precise for simultaneous quantitative analysis of amfepramone, amphetamine and methamphetamine.
The technique of gas chromatography developed here is useful and viable for testing involving routine toxicology, forensic toxicology and for certification analysis of the above-mentioned substances in non-biological materials. Furthermore, the technique might prove useful in industrial laboratories for quantitative analysis and quality control of amfepramone production.
The method’s flexibility, reliability, adaptability and affordability make it feasible for incorporation as mainstream law enforcement tool, enabling fast detection and quantification of illicit drugs, therefore having the potential to serve as a valuable tool for protection of the general population against drug abuse and intoxication.