The World Health Organization (WHO) has predicted a global amount of 19 million cancer case in 2025. After the lungs and liver, bone is the third most common site of tumour spread. The bone is a dynamic tissue that plays a critical role not only in structural support and movement, a reservoir for minerals and energy, but also houses of the bone marrow, which is the main site of postnatal hematopoiesis. A 54-year-old Indonesian woman presented to orthopaedist with complaints of sudden left hip pain and progressive difficulty in walking for three weeks. She reported severe pain that is exacerbated by any physical activity and movement. She has a medical history of breast cancer (pathologically confirmed in Sanglah General Hospital) which had undergone radiotherapy and chemotherapy a year ago. Outpatient left hip X-rays revealed lytic lesions at left acetabulum to left ilium, suggestive of metastatic disease. The established imaging techniques projectional radiography, skeletal scintigraphy, CT, MRI, and PET have undergone further development, with a resulting improvement in their diagnostic yield.
Cancer, X-rays, Acetabulum, Metastatic
The World Health Organization (WHO) has predicted a global amount of 19 million cancer case in 2025 [1]. After the lungs and liver, bone is the third most common site of tumour spread [2]. The bone is a dynamic tissue that plays a critical role not only in structural support and movement, a reservoir for minerals and energy, but also houses of the bone marrow, which is the main site of postnatal hematopoiesis [3]. The incidence of bone metastasis in all cancers is approximately 3-5%, and rises significantly to 19% in cancers affecting the prostate, lung and breast [2]. The early diagnosis of skeletal metastases has a major impact on the overall treatment strategy and is an important determinant of the course of illness and the quality of life [4]. The goal of diagnostic imaging is to detect skeletal metastases early, whenever they are suspected on the basis of clinical, laboratory findings, or in patients who are at high risk [4]. Delayed diagnosis has some negative effects on the prognosis and also enhances the risk of skeletal-related events (SRE), including fractures or spinal cord compression [5]. This study concern on early detection of metastatic bone disease (MBD) based on clinical symptoms and plain radiograph (X-ray).
A 54-year-old Indonesian woman presented to orthopaedist with complaints of sudden left hip pain and progressive difficulty in walking for three weeks. She reported severe pain that is exacerbated by any physical activity and movement. Her pain was minimally relieved with rest. The pain was slightly reduced with rest. She has a medical history of breast cancer (pathologically confirmed in Sanglah General Hospital) which had undergone radiotherapy and chemotherapy a year ago. Outpatient left hip X-rays revealed lytic lesions at left acetabulum to left ilium (Figure 1), suggestive of metastatic disease. She was diagnosed with suspected metastatic bone disease and treated with tramadol, eperisone, and methylcobalamin for her pain. An orthopedist recommended that she should be on bed rest indefinitely due to high risk of fracture as a result of his bone frailty. She was given the option to proceed with other examination, bone marrow biopsy, and appropriate treatment based on the results in bigger hospital which has those facilities.
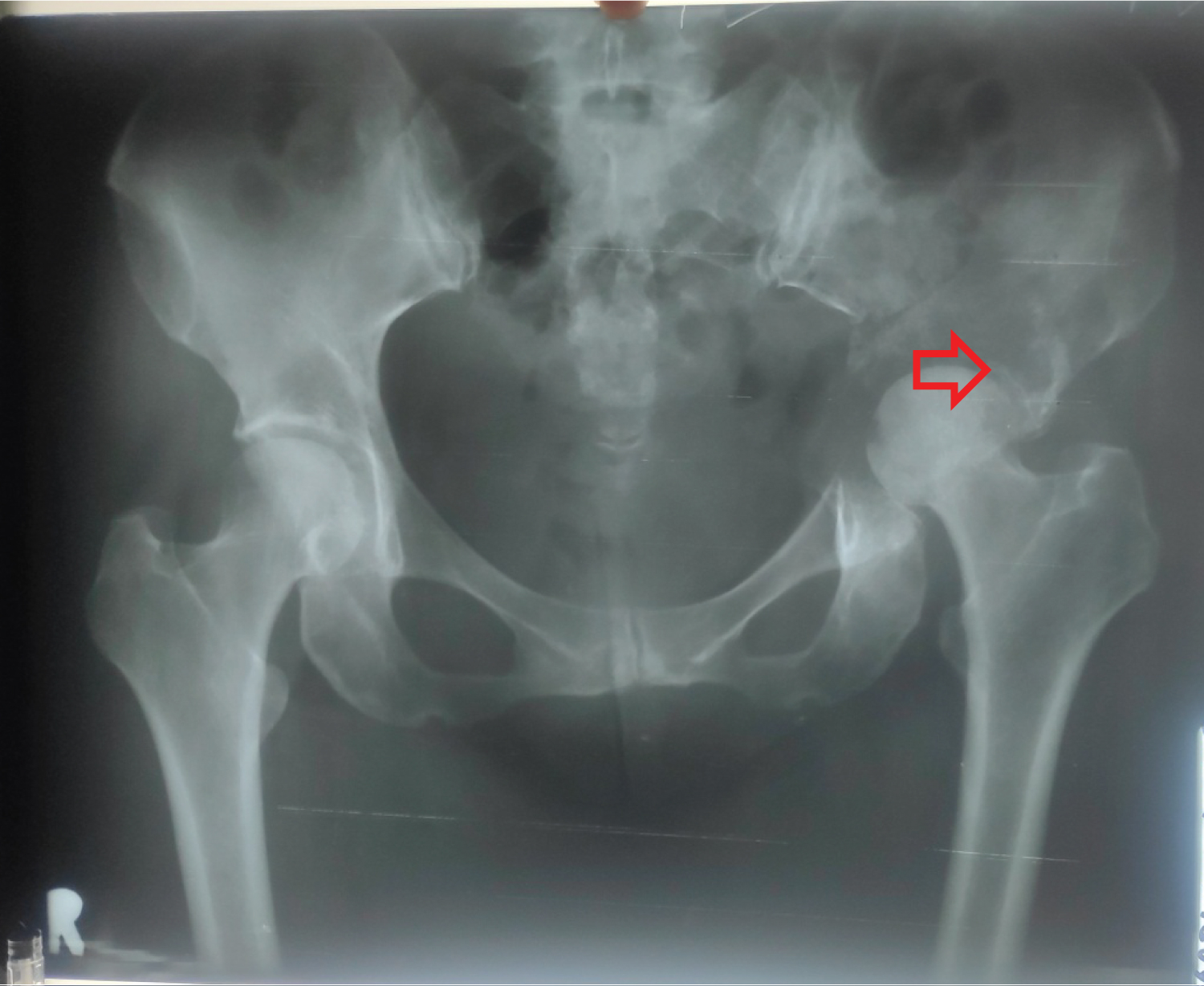 Figure 1: Lytic lesions at left acetabulum to left ilium.
View Figure 1
Figure 1: Lytic lesions at left acetabulum to left ilium.
View Figure 1
Breast cancer is one of the most common malignancies in the world [6]. Approximately 12% of women are diagnosed with breast cancer during their lifetime [6]. Bone is the most common site for breast cancer metastases, and 70% of patients with advanced breast cancer suffer from osteolytic bone metastases [6,7]. Bones are classified by their shapes, those are flat, short, long and irregular bones [1]. The outer part is covered with a fibrous layer and an inner osteogenic layer, the periosteum, and cambium layer which contains progenitor cells for the bone building cells, osteoblasts [1]. Behind the periosteum are densely packed tube-like structures called osteons (Haversian system) which consists of several layers (lamella) with small gaps (lacunae) in between, containing nutrient transportation cells, osteocytes, constituting 90 to 95% of the bone cells present in the mature human skeleton [1]. The packed osteons is the bone matrix, surrounding, and protecting the medullary cavity of the diaphysis, containing bone marrow, with a thin connective tissue membrane separating [1]. The hematopoietic lineage in the bone marrow is responsible for pre-osteoclastogenesis and mature osteoclast is a specialized macrophage with multiple mitochondria and lysosomes for bone degradation [1]. The cell-cell fusion process of pre-osteoclasts forming a mature osteoclast has a checkpoint, the stromal cells, which have the ability to interfere by secretion of Osteoprotegerin (OPG) [1]. The degradation of bone is initiated after maturation of osteoclasts and their allocation to the site-of-destruction, where they form a closed space, the resorption lacuna [1].
Bone is a common site of secondary tumor deposits because in its rigid, calcified, outer cortex, it has a richly vascular inner marrow of bony trabeculae, stroma, haematopoeitic tissue and fat [8]. The incidence of bone metastases is 65-75% in advanced metastatic breast cancer, 65-75% in prostate cancer, 60% in thyroid cancer, 30-40% in lung cancer, 40% in bladder cancer, 20-25% in renal cell carcinoma and 14-45% in melanoma [8]. In breast cancer, estrogen influences the bone microenvironment by creating and conditioning a favorable niche for colonization of breast cancer cells [8]. In this study, a 54-year-old Indonesian womanhas a medical history of breast cancer and has complaint of hip pain.Heightened awareness in the primary care setting of possible metastatic bone disease is essential in patients who present with musculoskeletal pain and a history of cancer or previous radiotherapy [9]. Subalakshmi, et al. found that from 36 patients more than 60% of the patient presented in average ranging from 40 to 60-years-old [10]. The most common symptom is pain and normally experienced around the site of the metastases, although sometimes can be referred to other areas from the affected structure [2]. This pain is caused by the release of cytokines and neuropeptides acting on the endosteum and subsequently the periosteum, as the tumour affects cortical bone [2]. Particular attention must be given to patient presenting with musculoskeletal pain and a background of cancer [2]. Commonly in patients who report functional pain, describing pain that comes on when completing everyday activities or experience difficulty weight bearing [2]. Importantly, simple analgesia has no effect on relieving this pain, which can help differentiate it from other musculoskeletal problems [2]. Functional pain is also a more accurate predictor of MBD compared with those who experience pain at rest [2]. Other symptoms of MBD are associated with complications of the disease and include hypercalcaemia, myelosuppression, metastatic spinal cord compression (MSCC) or pathological fractures [2]. Common sites for bonemetastasis include spine, pelvis, ribs, skull, and long bones [2,10]. Takagi, et al. (2015) found that 93 of the 286 patients (32.5%) with skeletal metastasis of unknown primary (SMUP) developed solitary bone metastases were located in the spine bones (44 cases (15.4%) cervical spine 3, thoracic spine 13 and lumbar spine 28), extremities (16 cases (5.6%) femur 4, humerus 4, tibia 8), pelvic bones (16 cases (5.6%) Iliac 13, pubis 1, ischium 2) and other bones (17 cases; 5.9%: skull 1, rib 6, clavicle 6, scapula 3, lower jaw 1), while 193 (67.5%) patients had multiple bone metastases [5].
Metastasis to bone can be occuredby direct extension, arterial or venous spread with the latter representing the most common form [11]. In circulation, the cancer cells entry into the venous circulation of the bone marrow is facilitated by the slow blood flow and the fact that hematopoietically active bone marrow is well vascularised [11]. Tumour cells produce adhesion molecules to bind to marrow stromal cells and bone matrix [11]. The normal remodelling process of bone provides chemotactic and growth factors which support these cancer cells once in place and make colonisation which interrupt normal bone cell turnover by releasing local cytokines and growth factors [11]. Certain tumours release factors which upregulate osteoclast activity like parathyroid hormone-related protein, tumour necrosis factor α or β, and other cytokines such as interleukin-1 and interleukin-6 which results in net osteolysis [11]. Other cancer cell types release factors like epidermal growth factor, transforming growth factor α and β, and insulin-like growth factors which upregulate osteoblasts resulting in net osteosclerosis [11]. Where osteolytic metastases tend to be aggressive [11]. Our patient has left hip X-rays revealed lytic lesions at left acetabulum to left ilium and has left hip pain. Plain radiographs are recommended to assess abnormal radionuclide uptake or the risk of pathological fracture and as initial imaging studies in patients with bone pain [11]. Osteolytic lesions demonstrate thinning of trabeculae and illdefined margins with the latter representing abnormal trabeculae between the centre of the lesion and the radiologically normal bone [11]. Conversely, sclerotic metastases classically appear as nodular, rounded, and fairly well circumscribed lesions secondary to thickened coarse trabeculae [11]. Although it uses ionizing radiation, it is an inexpensive, fast and accessible explorationthat allows detection of the presence of lytic lesions (with greater than 50% destruction of the mineralized bone), blastic lesions, mixed lesions or complications, pathological fractures [12]. Metastases measuring up to 1 cm in the spongiosa of a vertebral body or in the marrow of a long bone can be missed on plain X-ray, but pathological changes in cortical bone are detectable by plain X-ray even if they are only a few millimeters wide [4]. The MBD lesions are classified into lytic(lucent) or sclerotic (dense) metastasis, although features often coincide depends on the balance between osteoclastic activity (causing bone resorption), osteoblastic activity (causing bone deposition), as well as reactive bone changes (necrosis, fibrosis, or response to therapies) [13]. A bone lesionin a patient with any known primary malignancy should be considered a bone metastasis unless atypical features are present [13]. Early imaging and diagnosis of MBD can reduce morbidity and/or mortality [13]. On radiographs, the extent of bone destruction caused by the MBD lesion rather than the tumor itself is what is often depicted [13]. The diagnostic utility of plain films of the skull, spine, and pelvis is limited by superposition effects. In these areas, the sensitivity of plain films for bone metastases is only in the range of 44-50% [4]. The established imaging techniques projectional radiography, skeletal scintigraphy, CT, MRI, and PET have undergone further development, with a resulting improvement in their diagnostic yield [4]. For detecting metastases use surrogate parameters as a reference standard, because universal biopsies for histology (the theoretical gold standard) would be neither practicable nor ethical [4].
Patients with cancer involving the skeleton or those who have had previous radiotherapy to skeletal metastatic deposits are at particular risk of pathologic fracture [9]. After successful treatment of the primary tumor that often comprises surgery, with adjuvant chemotherapyand radiatiotherapy, and the administration of anti-hormonal drugs, patients frequently suffer from distant metastases even decades after a diseasefree interval [6]. Our patient has a medical history of breast cancer which had undergone radiotherapy and chemotherapy a year ago. Several landmark studies have suggested that the risk of pathologic fracture after radiotherapy can range from 13%-41%, 26% of patients develop disease progression at the bony site, and 35% of fractures develop at just only 6 months after radiotherapy [9]. When solid tumors metastasize to the bone they are often considered incurable [3]. However, the time from primary diagnosis to the development of clinical metastasis can range from months to decades, illustrating that tumor cells can leave the primary site and lay dormant for significant periods of time [3]. It remains unknown whether clinical dormancy results from tumor cell population dormancy, in which tumor cells continue to divide and die and/or are culled by the immune system, or cellular dormancy, in which tumor cells lay in a quiescent state for long periods of time [3]. The presence of disseminated tumour cells (DTCs) in bone marrow predicts poorer metastasis-free survival of breast cancer patients with localized disease [14]. DTCs persist in distant tissues despite systemic administration of adjuvant chemotherapy [14]. Many assume this is because the majority of DTCs are quiescent [14]. Chemoresistant DTCs occupy the perivascular niche (PVN) of distant tissues, where they are protected from therapy by vascular endothelium [14].
This report illustrate a patient who is suspected metastatic bone disease by plain radiograph with a medical history of breast cancer which had undergone radiotherapy and chemotherapy. Plain radiography is still useful for the immediate investigation of symptomatic bone pain and for the assessment of stability [4]. Although the sensitivity of plain radiograph for bone metastases is only in the range of 44-50%, early detection is required in this case to ensure effective management without any complications.