Schwannoma is a benign tumor, that mainly occurs in the nerve endings of the head and neck, and more rarely in the gastrointestinal tract. In the latter case, it appears as a submucosal tumor (SMT), which elective site is the stomach. Gastric schwannoma (GS) accounts for 0.2% of all gastric tumors and is mostly benign slow-growing, and very often asymptomatic. Its diagnosis can only be confirmed by a histopathological examination, which allows the distinction between a GS and other SMTs, particularly gastrointestinal stromal tumors (or GIST). Once a submucosal gastric mass's cells are positive for S-100 protein, the diagnosis of a gastric schwannoma is made. The best treatment remains complete resection with wide margins in the latter case.
We describe here the case of a 67-year-old woman, treated for a gastric schwannoma, revealed by abdominal discomfort for the previous 6 months. After full assessment including abdominal computed tomography (CT), upper digestive endoscopy, and gastric biopsy, we discovered a submucosal gastric lesion with benign macroscopic features, with no evidence of lymph nodes or metastatic involvement. She underwent atypical mid-gastric resection with gastro-gastric anastomosis. The final histopathological study with complementary immunohistochemical staining revealed a spindle cell tumor proliferation mimicking a gastrointestinal stromal tumor (GIST), with cells that were mainly positive for S-100 protein, and negative for CD 117 (or C-Kit). Given the good prognosis of the tumor, no adjuvant treatment was proposed apart from simple bi-annual clinical monitoring. With a follow-up of 28 months, the control is still satisfactory.
Gastric schwannoma, S-100 protein, Surgery, Prognosis, Case report
Schwannoma (also called neuroma or neurilemmoma) is a nerve tumor derived from Schwann cell. The latter produces myelin, which forms a protective sheath around nerve fibres (or axons), thus promoting nerve conduction. Schwannomas can develop anywhere along any nerve, more frequently in the head or neck area, and much more rarely in the gastrointestinal tract [1].
It is an essentially benign tumor. However, there are malignant forms: these are called malignant peripheral nerve sheath tumors (MPNST). Gastrointestinal schwannomas are a rare entity of gastrointestinal mesenchymal tumors, with only few clinical cases reported, as we gathered in (Table 1).
Table 1: Case reports on gastric schwannoma found in the literature. View Table 1
The Gastric Schwannoma (GS) develops at the expense of the nerve sheath of the submucous plexus of Auerbach or Meissner and corresponds morphologically in endoscopy to a submucous tumor.
Due to the absence of specific clinical and radiological signs, its diagnosis is based on the anatomopathological study which combines the morphological and immunohistochemical study which plays a crucial role in the diagnosis. This latter seeks specific immunostaining for the S-100 protein by the tumor and negativity for other antibodies such as C-kit, CD34, Desmin, and Smooth Muscular Actin.
Indeed, the anatomopathological examination makes it possible to distinguish the GS from the other SMT, and especially from the GIST which remains the main differential diagnosis. The only curative treatment remains carcinological surgery which provides an excellent prognosis [2].
We report here the case of a 67-year-old patient, followed at the Mohamed VI center for cancer treatment presenting a GS revealed by abdominal discomfort evolving at low noise.
We describe here the case of 67-year-old North African woman, with no pathological history, who presented through the previous 6 months diffuse abdominal discomfort, with no bowel disorders or nausea or vomiting.
An upper digestive endoscopy was performed on 07/04/2020 revealing suspicious submucosal budding at the level of the cardia. A laparotomy with atypical mid-gastric resection with gastro-gastric anastomosis was then performed. Macroscopic examination of the surgical specimen highlighted a white-beige nodular formation interposed between the mucosa and the gastric serosa, measuring 9 cm, well-circumscribed.
Histological examination showed a spindle cell tumor proliferation at the expense of the gastric submucosa, associated with a nodular lymphoid infiltrate in the peritumoral area (Figure 1). This proliferation consists of elongated cells with eosinophilic cytoplasm with poorly defined boundaries and oval nuclei (Figure 2). This aspect evokes first a GIST. But the immunohistochemical staining, highlighted an expression of the S-100 protein (Figure 3). In favor of nerve differentiation. On the other hand, the labelling for CD 117 (or C-Kit) turned out to be negative (Figure 4).
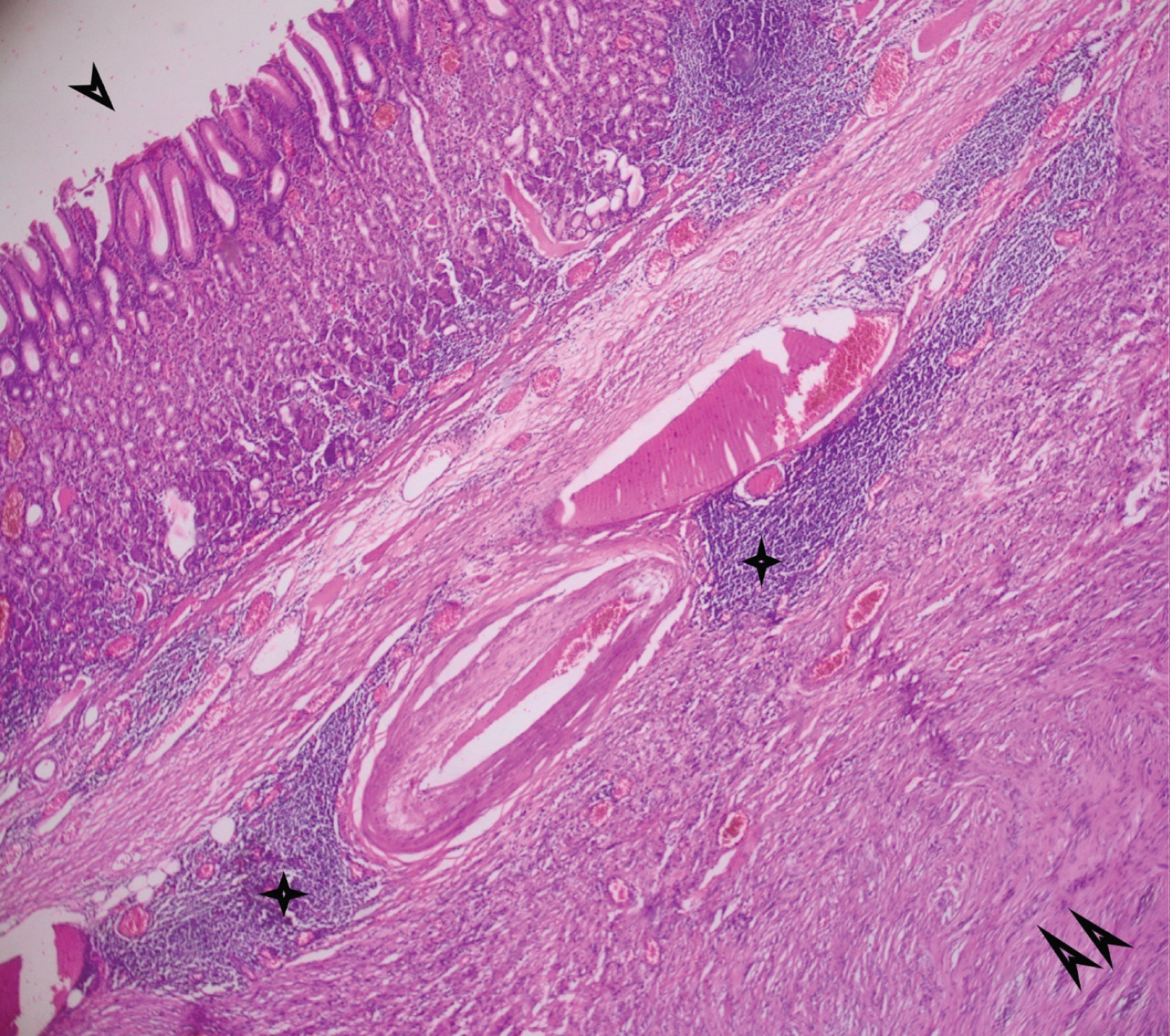 Figure 1: Fusocellular tumor proliferation (double arrow) in the gastric submucosa, associated with a nodular lymphoid infiltrate (star) peritumorally. (One arrow: gastric mucosa) [hematin-eosin stain, low magnification x 4].
View Figure 1
Figure 1: Fusocellular tumor proliferation (double arrow) in the gastric submucosa, associated with a nodular lymphoid infiltrate (star) peritumorally. (One arrow: gastric mucosa) [hematin-eosin stain, low magnification x 4].
View Figure 1
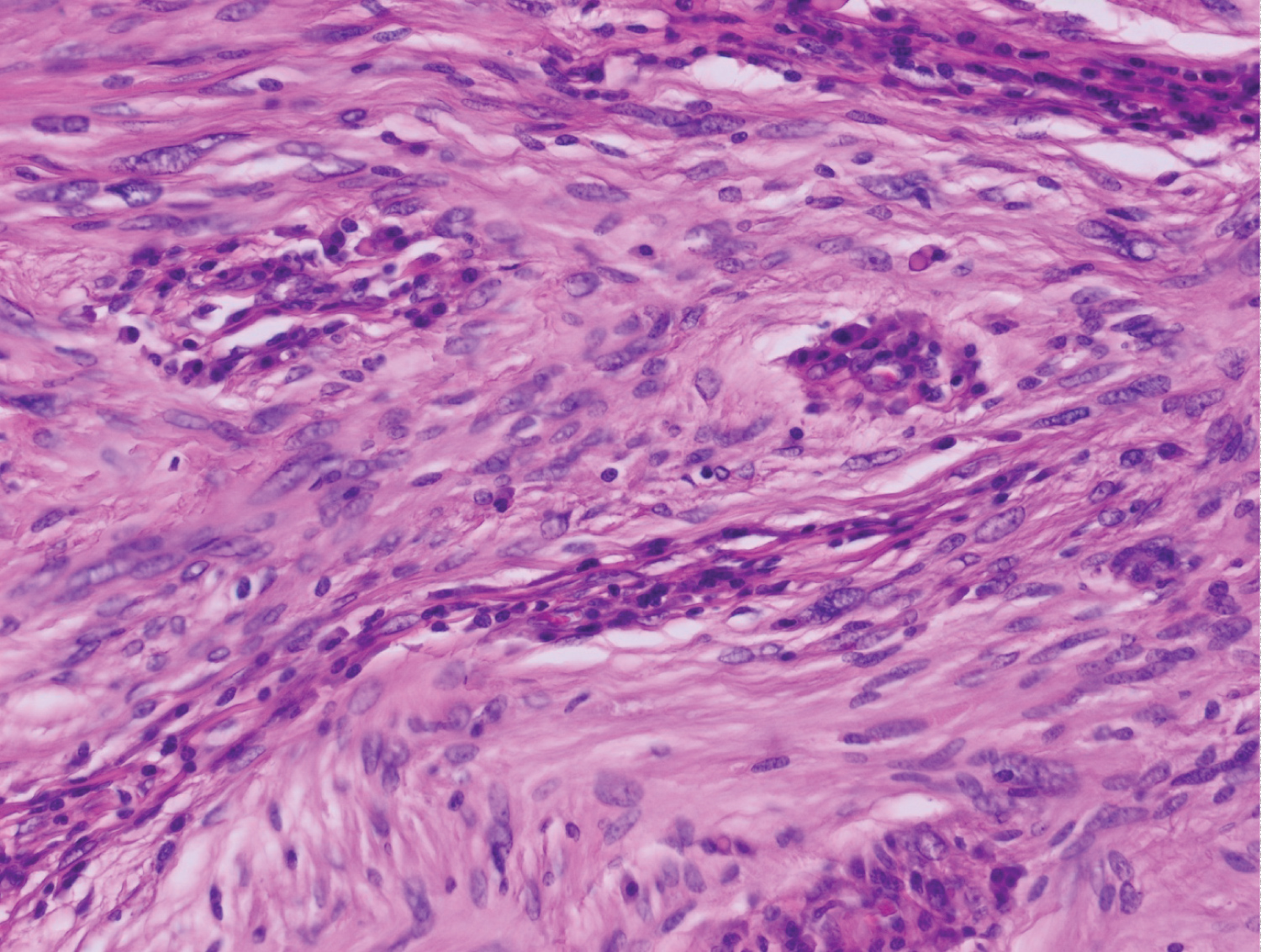 Figure 2: Spindle cell tumor proliferation made of elongated cells with eosinophilic cytoplasms with ill-defined boundaries and oval nuclei [hematin-eosin stain, high magnification x 40].
View Figure 2
Figure 2: Spindle cell tumor proliferation made of elongated cells with eosinophilic cytoplasms with ill-defined boundaries and oval nuclei [hematin-eosin stain, high magnification x 40].
View Figure 2
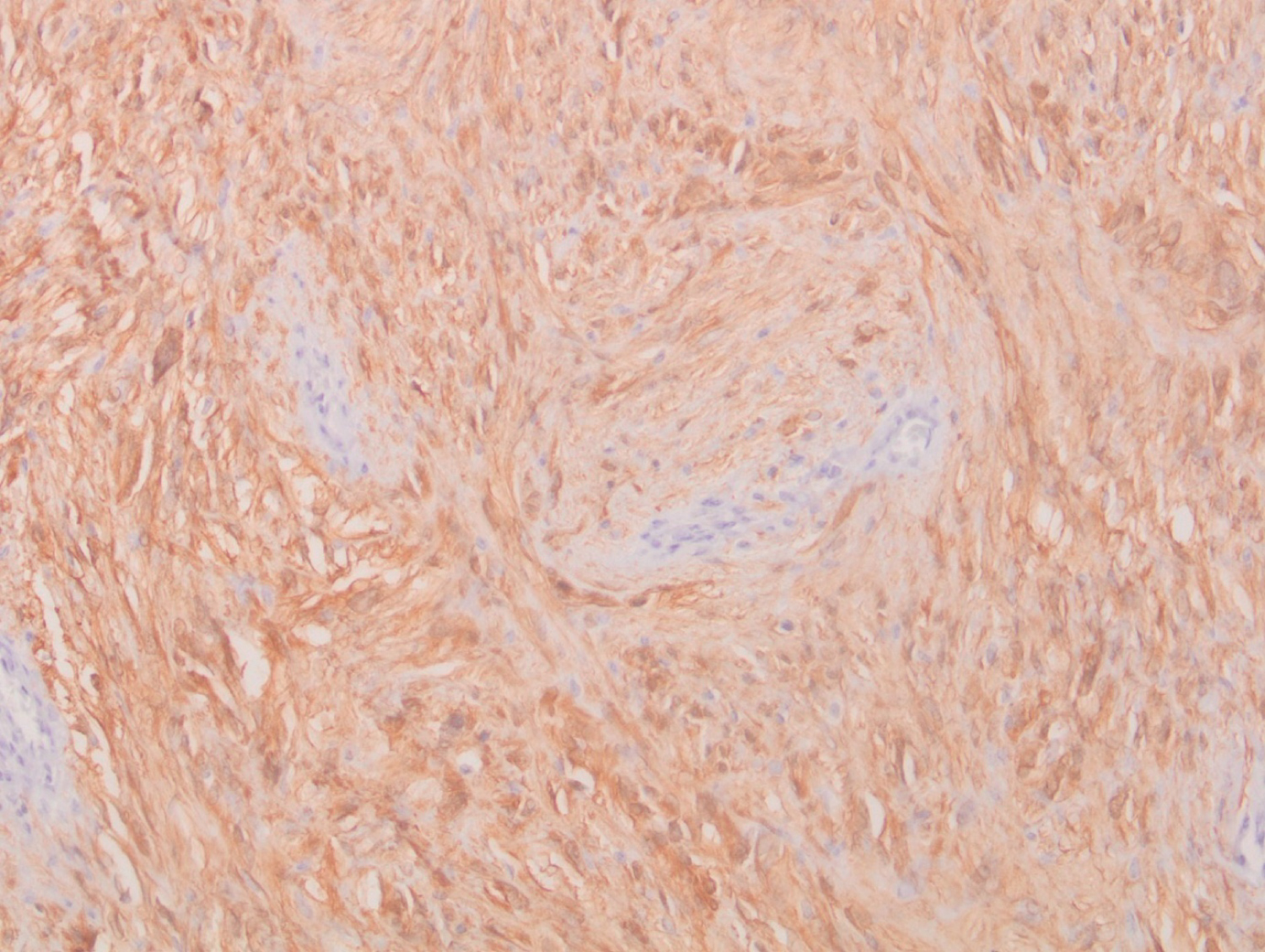 Figure 3: Diffuse expression of PS100 by tumor cells (immunohistochemical study).
View Figure 3
Figure 3: Diffuse expression of PS100 by tumor cells (immunohistochemical study).
View Figure 3
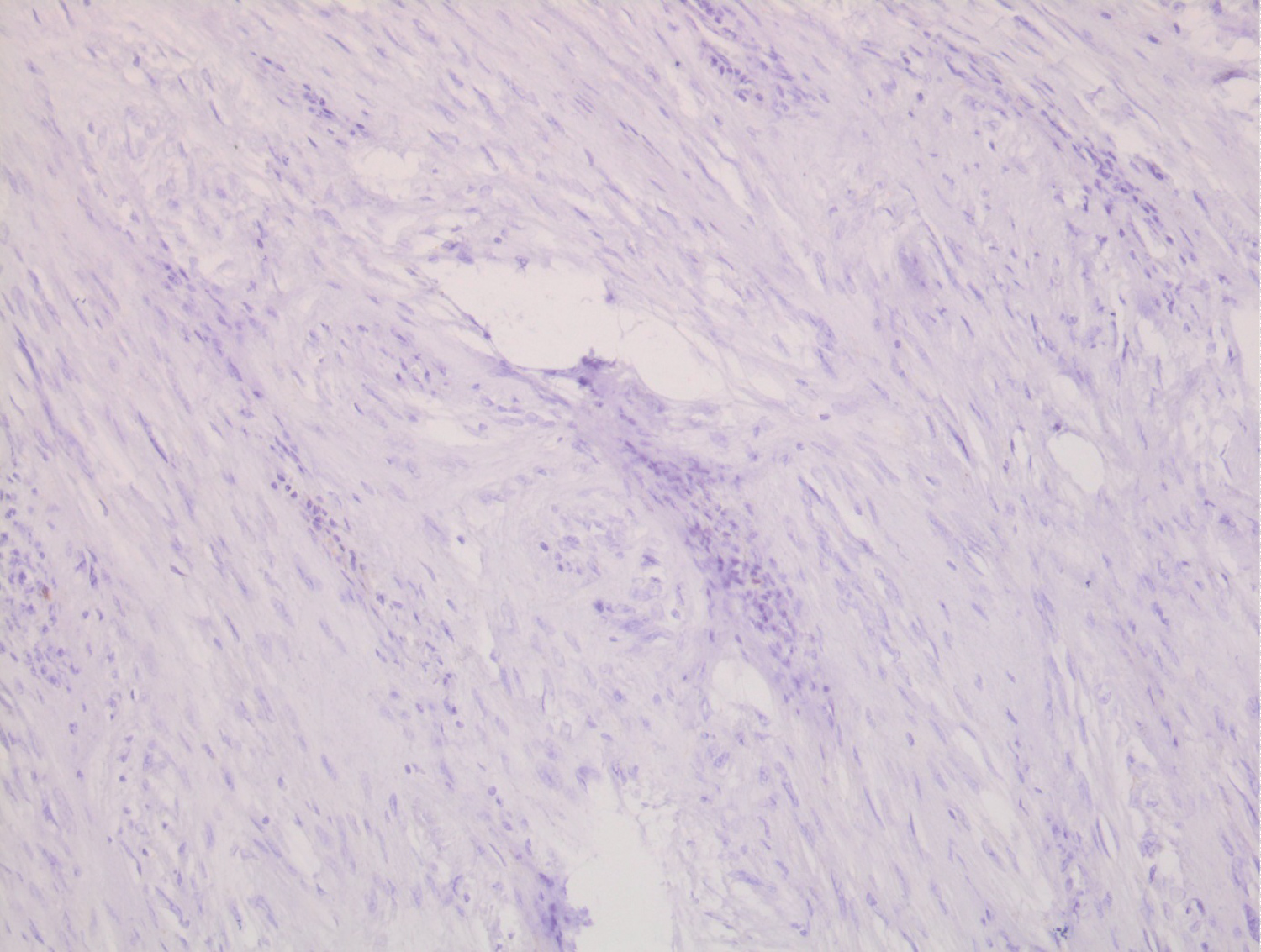 Figure 4: Absence of C-Kit expression by tumor cells (immunohistochemical study).
View Figure 4
Figure 4: Absence of C-Kit expression by tumor cells (immunohistochemical study).
View Figure 4
All these elements confirmed the diagnosis of a gastric schwannoma. A postoperative thoracic-abdominopelvic CT was performed revealing no primary or secondary progressive lesion. Given the good prognosis of the tumor, and wide surgical resection margins, no adjuvant treatment is proposed apart from simple bi-annual clinical monitoring. With a follow-up of 28 months, the control is still satisfactory.
The epidemiology of GS is poorly understood. Indeed, it could not be reliably separated from GISTs. The identification of true nerve tumors is relatively recent.
Voltaggio, et al. estimated that the ratio of gastric GIST to GS is approximately 45 to 1 [3]. These tumors occur at any age, but generally between the ages of 40 and 60, with an average of 58 years [4].
Depending on the series there is a slight female predominance [4-6]. The GS is usually in the form of an isolated lesion and is usually localized in the body of the stomach [7,8].
The genesis of this tumor remains poorly defined due to the scarcity of series focusing on it. However, neurofibromatosis could be implicated according to some cases published. This requires the search for clinical signs of associated neurofibromatosis, to detect a family susceptibility.
The risk of developing cancer, including malignant schwannoma, would be multiplied by 4 in the case of NF1 type neurofibromatosis compared to the general population [9].
Some authors mention the involvement of the loss of expression of merlin (or schwannomine), a protein coded by the tumor suppressor gene NF2 (located on chromosome 22) in the carcinogenesis of schwannoma [10,11].
The schwannoma is a firm tumor generally encapsulated, spherical or ovoid, developed along a nerve, and sometimes adheres to the nerve trunk itself, pushing back the nerve fibers but never invading them [12,13]. Its implantation base can be sessile or pedunculated [13]. It is pearly-white, gray, or even yellowish in the event of fat infiltration, with necrotic or hemorrhagic areas depending on the case. Its size varies from 1 to 15 cm depending on the literature.
A useful histological clue for its diagnosis is the presence of a peritumoral lymphoid infiltrate [14]. Cells have spindle-shaped nuclei. Finally, Positive immunostaining for the S-100 protein confirms the diagnosis [15]. Immunohistochemistry constitutes real progress in the diagnosis of SMT thanks to the increasing number of good-quality antibodies available [16,17].
Other antigens that can be tested are:
- GFAP ‹‹ Glial Fibrillary Acidic Protein››,
- Leu-7‹‹ Myelin Associated Glycoprotein ››
- CD117
- CD34
- Desmin
-Smooth muscle actin (SML)
-Striated Muscle Actin (SMA)
-Myosin.
Schwannomas are always strongly positive for the S-100 protein, inconstantly positive for GFAP and Leu 7, and rarely positive for CD34. On the other hand, they are negative for CD117 (or c-KIT), Desmin, SML and Myosin [18].
Clinically, GS remains asymptomatic for years in 30 to 50% of cases, and its discovery is often incidental during imaging, endoscopy, or surgery performed for another pathology [19]. Indeed Choi, et al. [20] evaluated the growth rate of GS based on follow-up CT. Tumor doubling time was approximately 5 years.
Abdominal pain remains the most frequent revealing mode, manifested by vague signs such as discomfort, heaviness, or feeling of abdominal fullness.
Other signs may accompany these painful phenomena:
- an alteration of the general state.
- an anemic syndrome, related to occult bleeding.
- nausea and vomiting.
More rarely, the patient can suffer from mechanical complications such as bowel obstruction, pylori-duodenal intussusception of an antral tumor [21], torsion, gastric volvulus on pedunculated schwannoma, jaundice due to compression of the biliary tract. Exceptionally, GS can be revealed by a rupture leading to acute abdominal pain with septic shock.
No specific biological abnormality has been reported in the literature. However, hypochromic microcytic anemia associated with occult bleeding is frequently found.
The endoscopic aspects of GS are not specific. When detectable, the tumor most often presents as a protruding, rounded submucosal mass. It sits mainly in the gastric body. Furthermore, an endoscopy allows biopsies to be performed even if they are not contributive to the diagnosis due to the normal mucosa covering the submucosal lesion.
Echo-endoscopy or EUS (Endoscopic Ultrasonography) is an examination that combines endoscopy with ultrasound. It is the most efficient examination for the exploration of SMT and allows deeper biopsies than conventional endoscopy. In most series, the GS appears as a rounded lesion, located at the level of the muscular or the submucosa, hypoechoic and homogeneous. Unfortunately, these signs are not specific because they can also be found in GISTs [22].
Endoscopically guided fine-needle aspiration is not routine as recommended by the National Comprehensive Cancer Network (NCCN) for resectable SMT due to the risk of tumor rupture and spread, which is associated with poor prognosis.
Abdominal CT is an essential morphological examination, especially if surgery is planned. The GS usually appears as a well-limited submucosal mass, of tissue density, hypodense, and homogeneous, with diffuse enhancement after injection of contrast product.
Cystic transformation, cavity formation, necrosis, or calcification are more rarely observed phenomena [23]. In some cases, reactive perigastric lymphadenopathy is found [24].
No specific pathognomonic sign has been formally identified, but some peculiarities have been reported in an English series, making it possible to distinguish GS from its main differential diagnosis: GIST [25]. These peculiarities are grouped in (Table 2). However, central ulceration can be observed in 25 to 50% of cases due to ischemic phenomena of the covering mucosa [26].
Table 2: Main radio-clinical differences between GS and GIST. View Table 2
The MRI (Magnetic Resonance Imaging) appearance of GS has rarely been documented. It makes it possible to identify the layer of origin of the process (muscular or submucosa). The lesion is of low to medium intensity on T1 and high intensity on T2 with homogeneous enhancement after injection of gadolinium [27].
Unfortunately, even position emission tomography coupled with a scanner (PET-CT) cannot differentiate a malignant schwannoma from a benign schwannoma or other SMTs, since they all uptake 18-FDG equally [28-30].
The reason for the high 18F-FDG uptake in benign tumors like schwannomas is unclear. Beaulieu, et al. [31] reported that this high uptake of FDG in schwannomas may result from the activity of Schwann cells to transport glucose for axonal repolarization.
To this date, the only effective treatment is surgical excision, which is both diagnostic and therapeutic [32]. Laparoscopic and endoscopic surgery is increasingly used for gastric SMT [33]. Kikuchi, et al. [34] developed a surgical technique known as "closed" laparoscopy which avoids the risk of spreading tumor cells into the abdominal cavity. Despite the development of endoscopic therapeutic techniques, intramuscular tumors measuring more than 3 cm require conventional surgical management, due to the risk of perforation [35].
Radiotherapy has no place in therapeutic management. It is indicated only in palliative intention, in the event of painful metastatic evolution. Similarly, no systemic treatment is indicated. However, the growing interest in molecular therapies would justify a series to determine their roles in the treatment of malignant schwannoma.
The usual course is towards recovery as long as surgical excision is feasible. Hong, et al. [36] studied 137 cases of benign GS and identified no recurrence or metastatic relapse cases during a follow-up period ranging from 1 to 336 months.
This excellent prognosis makes post-therapeutic follow-up by a morphological assessment obsolete. Indeed, a simple clinical follow-up alternated between oncologist and surgeon is preferable.
A few rare cases of malignant GS have been reported in the literature [37,38]. They are extremely rare and can only be distinguished from benign schwannomas based on histopathological examination of biopsy specimens.
Nevertheless, Bao-Guang Hu and coll [28] gathered 221 cases of GS, including 211 benign and 10 malignant, and concluded that a follow-up of at least 5 years would be necessary for malignant GS to avoid a disease relapse.
The GS is a tumor, most often benign with slow and uniform growth. Because of this, the diagnosis is usually delayed. They represent a therapeutic challenge, due to difficulties in obtaining a preoperative diagnosis, due to the absence of specific clinical, radiological, and endoscopic signs.
A definitive diagnosis can only be established by histopathological and immunohistochemical analysis of the surgical specimen. The treatment of choice remains complete surgical resection with a negative margin.
The prognosis of the patients is excellent, justifying only clinical follow-up, to avoid the inconveniences linked to carrying out a repeated morphological assessment.
The authors would like to thank the patient for allowing us to report her clinical information and data.
Not applicable.
Written informed consent was obtained from the patients for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
All data generated or analyzed during this study are included in this published article.
The authors declare that they have no competing interests.
In Morocco, there is no national ethical committee. We have law on biomedical research and the protection of persons involved in research is under preparation at the Ministry of Health, which doesn't concern our work.
None.
The authors confirm their contribution to the paper as follows:
FO did the follow-up of the patient and took care of writing the clinical case.
SB performed the histopathological study of the surgical specimen and sent us all the iconography with its explanations, and supervised this work.
SS, NT, HJ, NB, and ZB validated the final version of the manuscript.