Methotrexate induced Stevens-Johnson syndrome (SJS) is rare but life-threatening cutaneous reaction. This is a case report of two 14-year-old girls with methotrexate (MTX)-induced Stevens-Johnson syndrome (SJS). Both girls received chemotherapy with MTX in the treatment of osteosarcoma and then developed SJS. They showed cutaneous and mucosal erosions, macular and purplish rash, and ulceration in the cutaneous and mucosal areas. The full spectrum of all mucous membranesinvolved is less than 10% of the body surface area in the two patients.
The diagnosis of SJS is based on clinical features and there are no universally accepted diagnostic criteria. Prompt withdrawal of causative drugs should be a priority and the re-exposure of culprit drug is not recommended.
Corticosteroid at a dose of 1 mg / kg / day was administered to them and they both recovered from SJS. MTX is known as a drug that can induce SJS. So, it is rare but is not extremely rare.
Stevens-Johnson Syndrome, Osteosarcoma, Methotrexate
Methotrexate is a folic acid antagonist that inhibits the reduction in folate supply and the proliferation of tissue cells [1]. Since the proliferation of malignant cells is greater than that of normal cells, Methotrexate can slow their proliferation without causing irreversible damage to healthy tissue hence the interest of its use in the treatment of several neoplasms.
Methotrexate is a drug that must be used with caution due to its possible side effects such as inflammation of the oral mucosa, blood-toxicity and disturbance of liver and kidney function [2]. Although its side effects are generally benign, there are some severe adverse effects such as Stevens- Johnson-Syndrome (SJS) which is a life-threatening mucocutaneous reaction rarely complicating Methotrexate administration [3,4]. We reported two cases of Steven-Johnson Syndromeinduced by high doseMethotrexateas neoadjuvant chemotherapy for a high-grade osteosarcoma treated at the medical oncology department at CHU Habib Bourguiba Sfax, Tunisia. The two patients were treatedaccording to the OS 2006 protocol. The treatment is based on high-dose Methotrexate cures (12 g/m2), each injection is followed by rescue treatment with folic acid and alkaline hyper hydration at weeks 1,2,3,7,8,12, and 13 and courses of Etoposide and Ifosfamide at weeks 4 and 9.
A 23-year-old female presented to us with a 24-hour history of fever associated with a non-productive cough. The fever was of low-grade intermittent nature and accompanied by chills but no rigors. She had 5-6 episodes of loose watery stools daily for 5 days. They were of small volume, not foul smelling or difficult to flush and had no blood or mucus. There was no history of recent food consumption from outside her home or of recent antibiotic use.The first patient is a 14-year-old girl, with no medico-surgical history; She is followed for an osteosarcoma of the upper extremity of the right humerus with pulmonary metastasis.
On the 7thday of the 7thweek, the patient consulted with, plantar erythema grade 1 (Figure 1); grade 2 mucositis and periorbital erythema without conjunctival involvement (Figure 2). The biology exams noted 32 time’s normalcytolysis and acute kidney failure. Methotrexate toxicity was suspected. The patient was treated with alkaline hyper hydration, rescue treatment with folic acid, and Fluconazole for her mucositis.
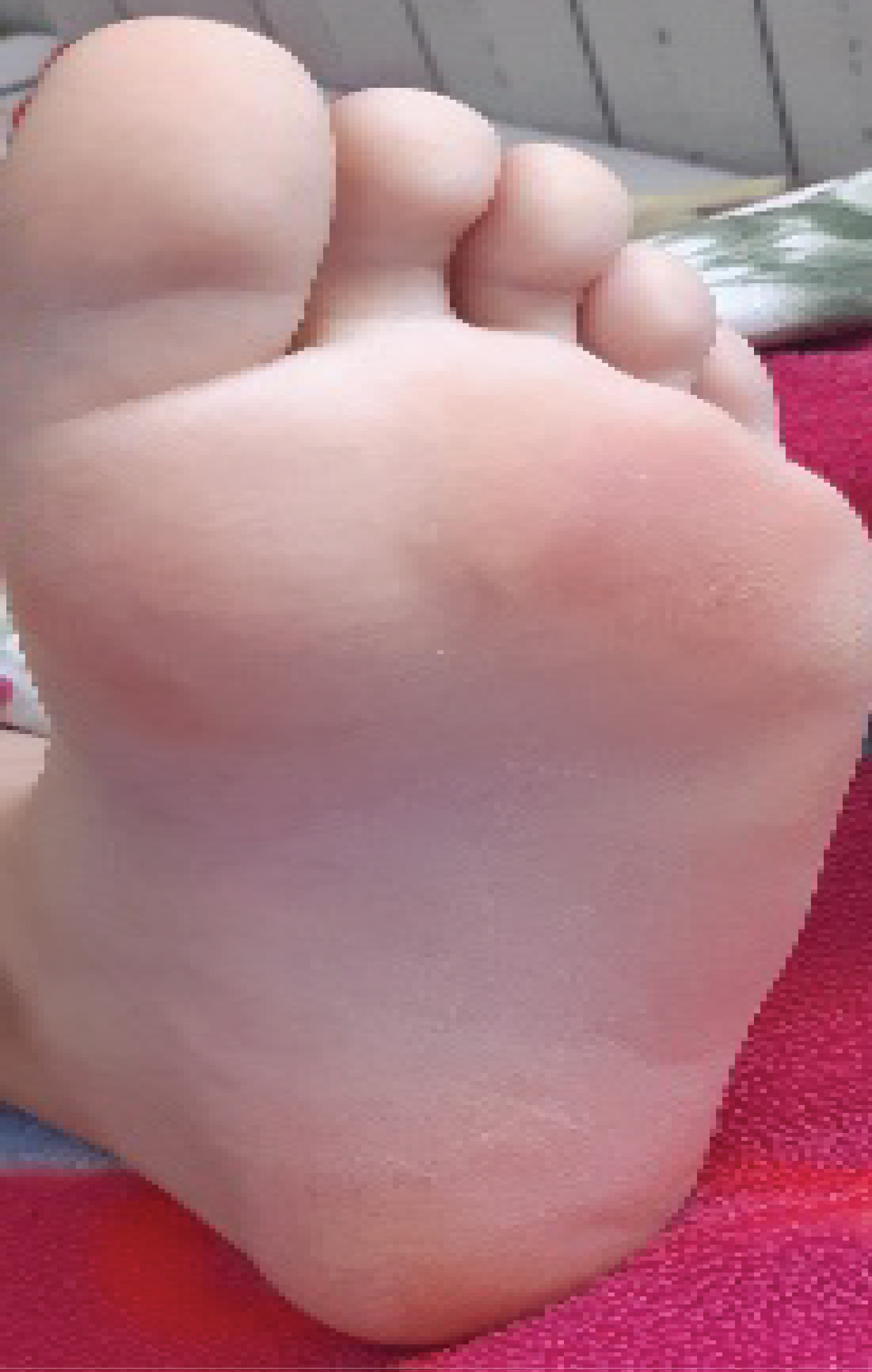 Figure 1: Planter erythema.
View Figure 1
Figure 1: Planter erythema.
View Figure 1
 Figure 2: Periorbital erythema.
View Figure 2
Figure 2: Periorbital erythema.
View Figure 2
The evolution was marked by the aggravation of the symptoms. At the 12thday of chemotherapy, she presented erosive hemorrhagic cheilitis with multiple intraoral erosion (Figure 3), peri and endo-mammary erosions and crusts, a slight macular rash in the trunk (Figure 4), lesions cockade on the level of the backs of the hands (Figure 5) and painful perianal ulceration (Figure 6). Atbiology exams, we noted severe pancytopenia and a slight improvement in hepatic cytolysis.
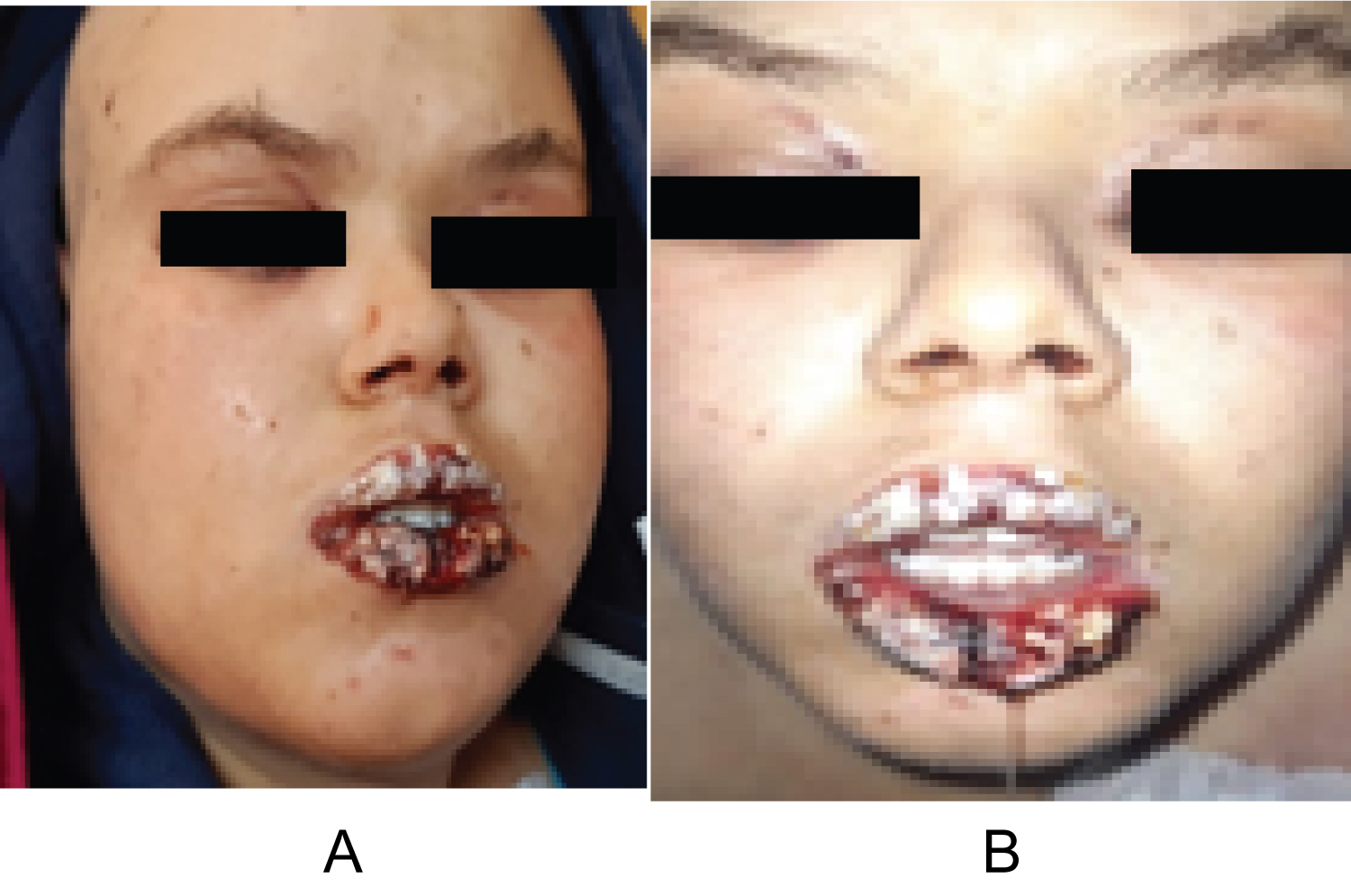 Figure 3: Erosive hemorrhagic cheilitis (a,b).
View Figure 3
Figure 3: Erosive hemorrhagic cheilitis (a,b).
View Figure 3
 Figure 4: Lesions cockade on the level of the backs of the hands.
View Figure 4
Figure 4: Lesions cockade on the level of the backs of the hands.
View Figure 4
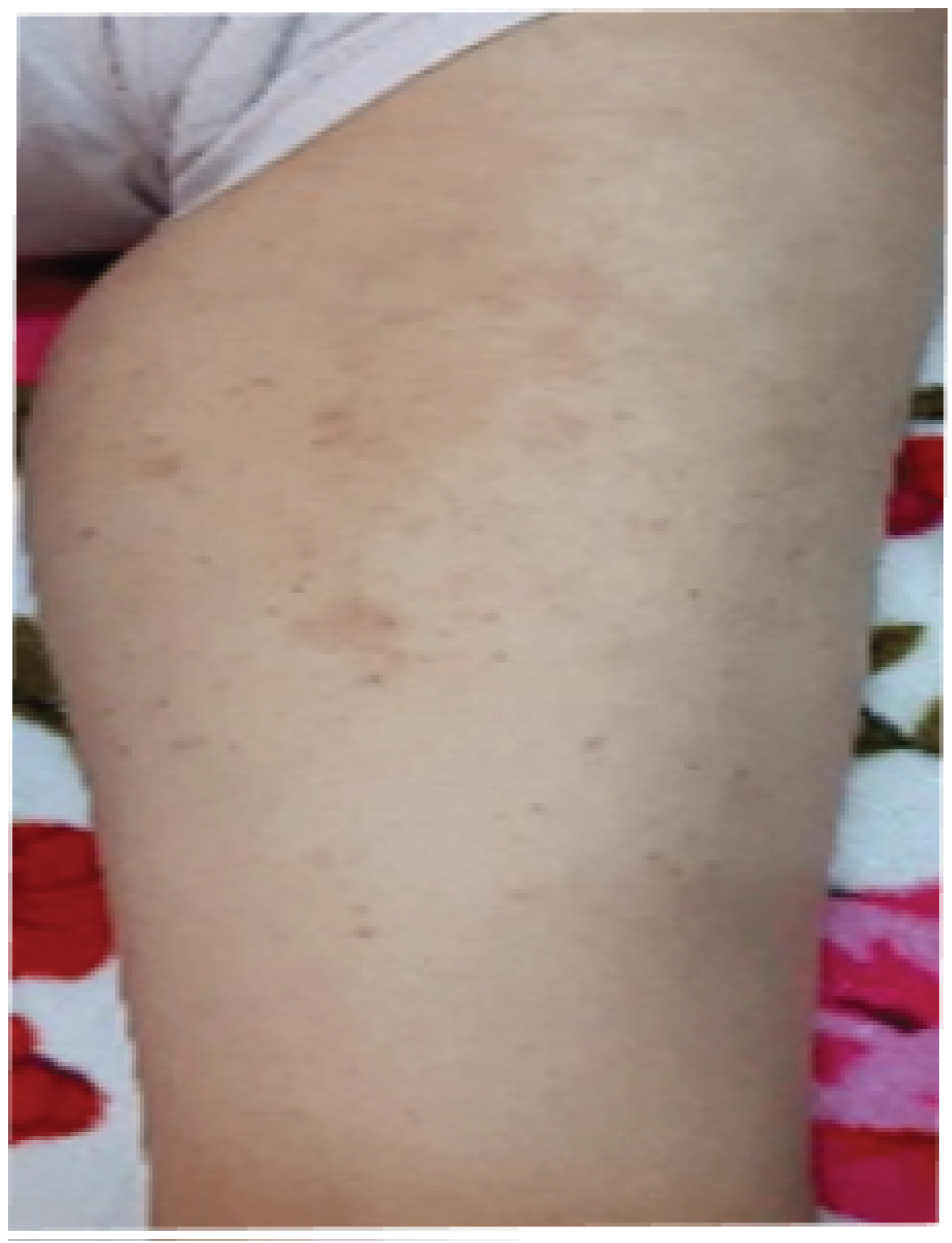 Figure 5: Macular rash.
View Figure 5
Figure 5: Macular rash.
View Figure 5
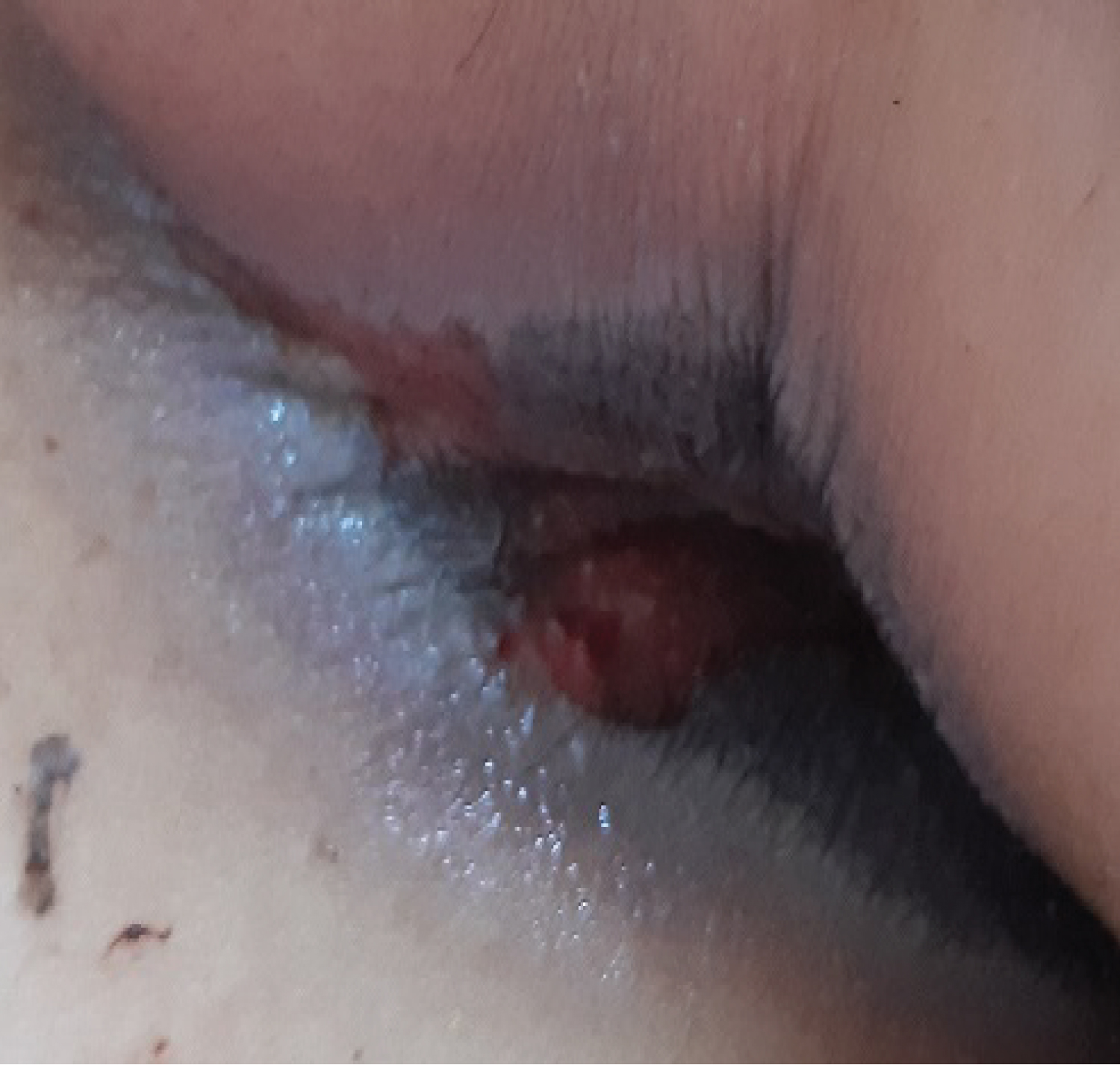 Figure 6: Perianal ulceration.
View Figure 6
Figure 6: Perianal ulceration.
View Figure 6
The diagnosis of SJS was suspected and the contraindication to re-administration of Methotrexate has been decided. The patient received systemic corticosteroid therapy at a dose of 1 mg/kg/day with mouthwashes. Improvement of the symptomatology anda complete disappearance of the lesions 15 days after corticosteroid therapy initiation were noted.
She is a 14-year-old girl with no medico-surgical history; she is followedfor alocalized osteosarcoma of the right femur. At the 10th day of the first cycle of high-dose Methotrexate, the patient consulted for fever, sore in the mouth and throat and cutaneous reaction. The clinical exam notedgrade IV mucositis, skin erosion especially in the elbows (Figure 7), an ulceration in the lining of the mouth (Figure 8), a purplish rash in the limbs (Figure 9) and squamous lesions in the extremities (Figure10). The biological examsshowed grade IV neutropenia, hepatic cytolysis 9 times normal value and an acute renal failure.
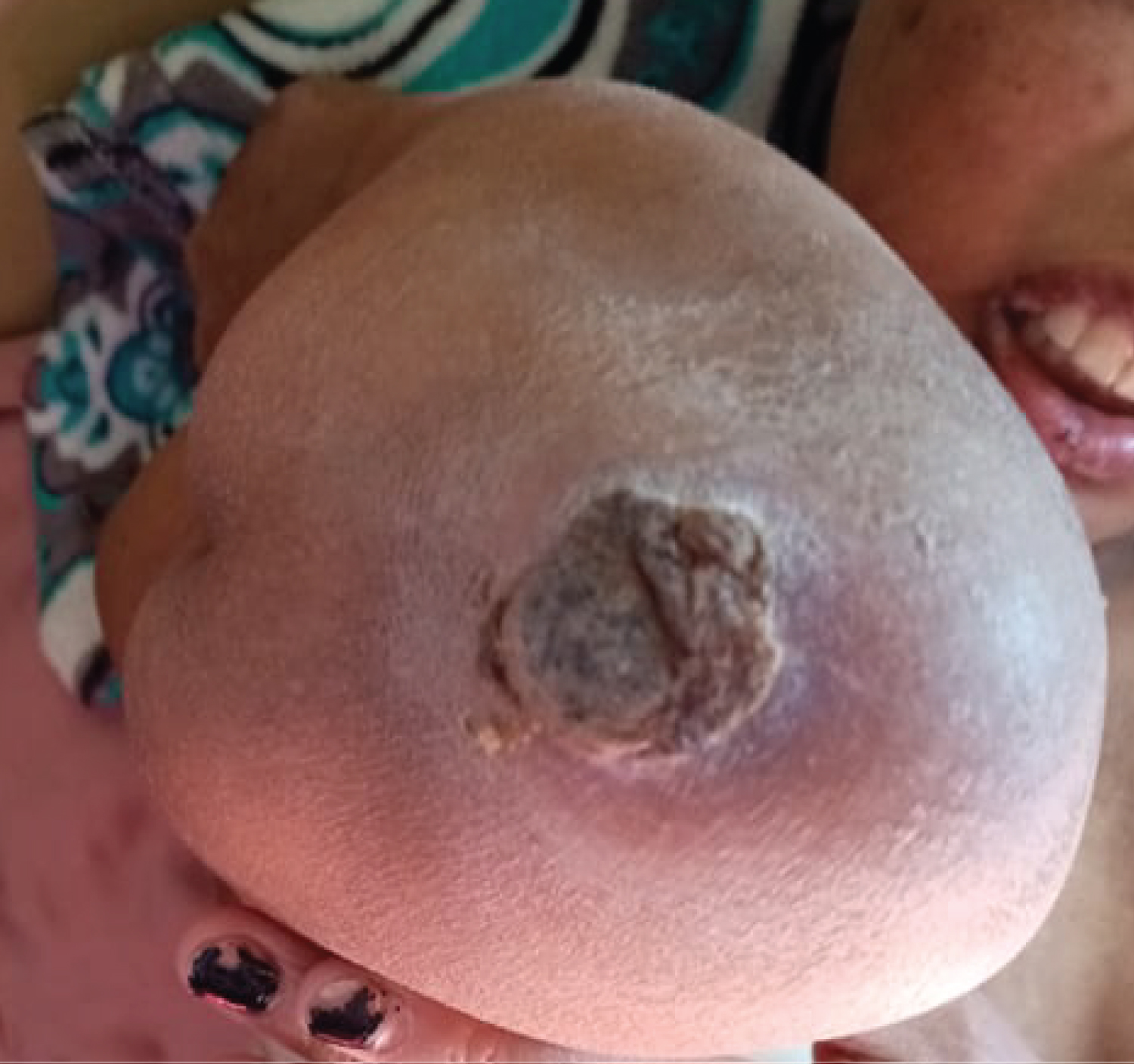 Figure 7: Elbow erosion.
View Figure 7
Figure 7: Elbow erosion.
View Figure 7
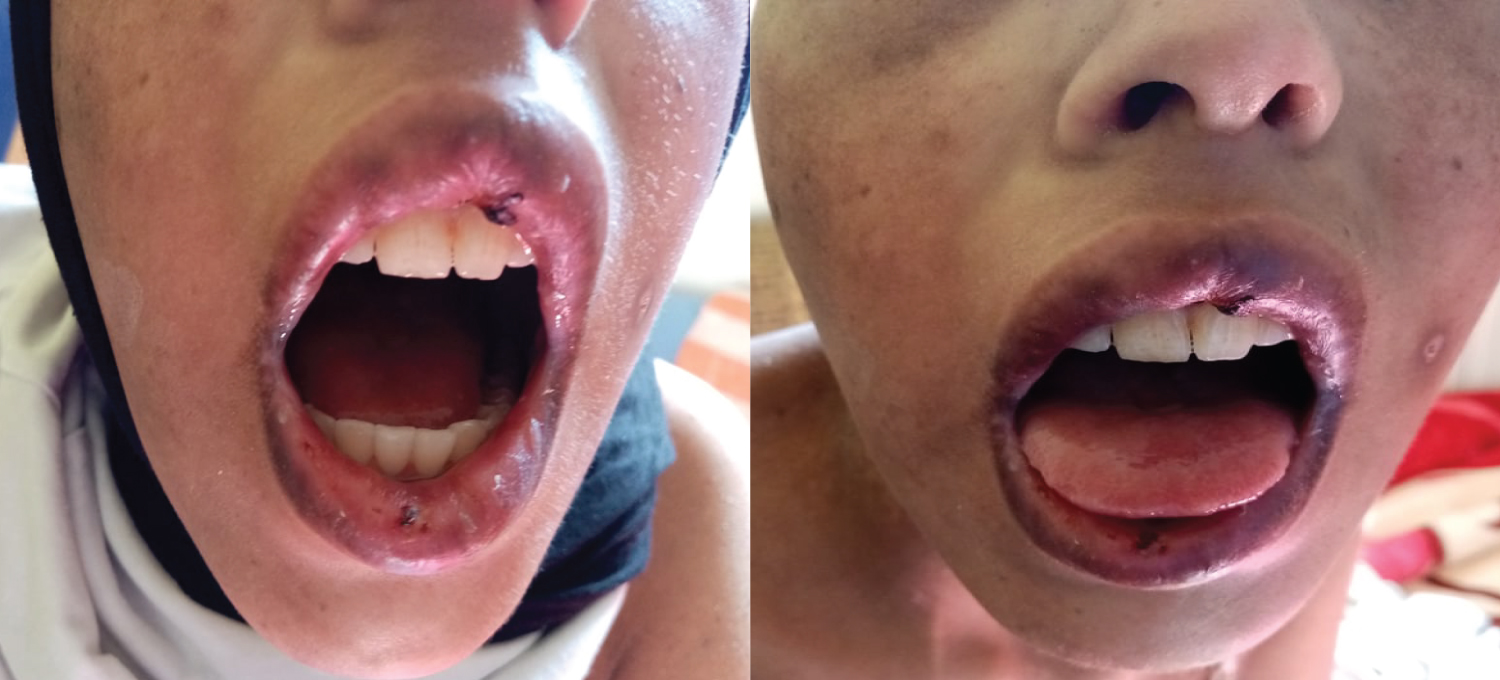 Figure 8: Ulceration in the mouth.
View Figure 8
Figure 8: Ulceration in the mouth.
View Figure 8
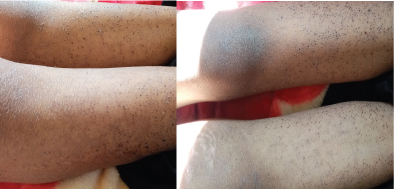 Figure 9: Purplish rash in the limb.
View Figure 9
Figure 9: Purplish rash in the limb.
View Figure 9
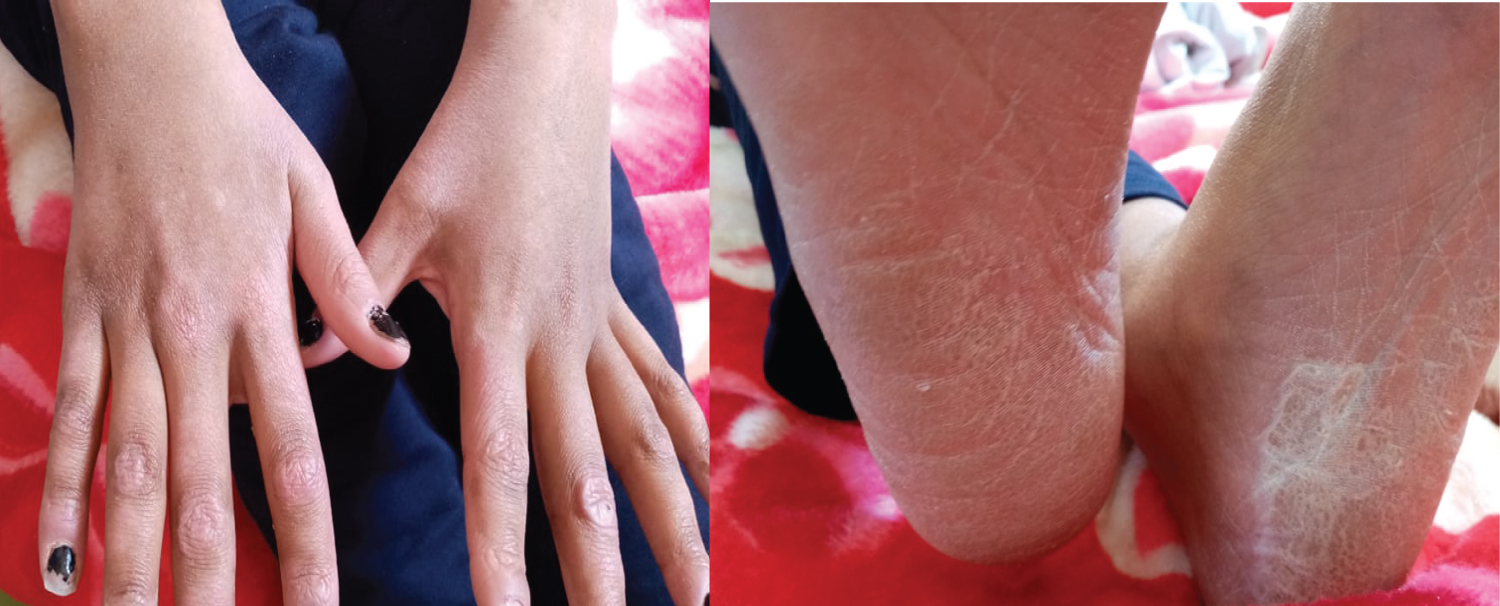 Figure 10: Squamous lesion in the extremities.
View Figure 10
Figure 10: Squamous lesion in the extremities.
View Figure 10
The diagnosis of SJS was suspected associated with febrile neutropenia, hepatic, and renal toxicity due to Methotrexatechemotherapy. The patient was treated with broad spectrum antibiotherapy, corticosteroid at the dose of 1 mg/kg, mouth wash, alkaline hyperhydratation and rescue with folic acid.
The evolution was characterized by the resolution of the lesions and the decision was to avoid the re-administration of Methotrexate and then the patient was referred for surgery of the primary tumor.
Steven-Johnson Syndrome is a rare but life-threatening mucocutaneous reactions [5]. Recently, there is an increased number of reports being published on Methotrexate (MTX) related SJS. We report some of these cases [1,3] [6-8] (Table 1).
Table 1: Cases report of SJS related to MTX.View Table 1
Clinically, this syndrome is characterized by acute skin blisters, scalding of the skin, and mucous membrane erosions [6] [9-12]. The detachment spectrum is considered to be < 10% [13] in the SJS. The skin is often tender to the touch, with lateral pressure producing shedding of the epidermis from the dermis which is called as the Nikolsky’s sign. Multiple organ systems such as cardiovascular, pulmonary, gastrointestinal, and urinary systems may be affected [1,3,6] [ 14-16].
Because of the mechanism of action of MTX, these cases are different than that of the “traditional” SJS. This syndrome is usually considered a hypersensitivity reaction [9,15,16], but with MTX it might be due to the fact that the actual cells in the basal layer are unable to reproduce at an adequate rate. Thus, widespread epidermal necrosis occurs as a result of keratinocytes’ apoptosis. This apoptosis is caused by the binding of FAS (CD95), a membrane receptor present in keratinocytes, with its FAS ligand (CD95L) [9].
Drug exposure is the cause of large majority of cases. The most commonly implicated drugs are Allopurinol, Trimethoprim-Sulfamethoxazole and other Sulfonamide-antibiotics, Aminopenicillins, Cephalosporins, Quinolones, Carbamazepine, Phenytoin, non-steroidal anti-inflammatory drugs, and phenobarbital anticonvulsants [9,11,16,17].
Our two cases developed the full spectrum of the disease with involvement of all mucous membranes. The skin is involved in less than 10% of the body surface area. Steven-Johnson Syndrome-like exanthema in our two patientswas related to Methotrexate administration. It occurred after fourthexposure to the drug in the first patient and the first exposure to the drug in the second one.
The severe cutaneous manifestations of this disease spectrum may often lead to overlooking of the ocular sequelae, which are very common and may lead to loss of visual acuity [18]. Fortunately, both patients had no symptoms of the eye (conjunctivitis). Diagnosis of Steven-Johnson Syndromeis based on clinical features and there are no universally accepted diagnostic criteria.
Prompt withdrawal of causative drugs should be a priority with early initiation of supportive treatment which includes monitoring of fluids and electrolytes, barrier nursing care, nutritional support and control of infection [6]. Corticosteroids has been proposed as a treatment strategy [9,19,20]. The main mechanism of intended action is the modification of almost all components of the inflammatory and immune response. But the timing, corticosteroid type dosage and duration remain unclear. Our patients were treated with intravenous corticosteroids successfully.
Steven-Johnson Syndrome is rare clinical conditions with high mortality and morbidity rate. Early diagnosis and administering appropriate treatment mayimprove the prognosis. Although the re-exposure of culprit drug after SJS is not recommended. The main intention of these cases report of Methotrexate induced Steven-Johnson Syndrome is to make the clinicians as aware of this occurrence.