Background: Bleomycin is chemotherapeutic agent that has been used for cancer treatment for several years. However, potentially life-threatening lung toxicity is a major concern limiting its use.
Case report: This is a case of a 64-year-old male presenting sub acutely with gradual onset of exertional shortness of breath and cough after completing 4 cycles of ABVD regimen for Stage 2b Hodgkin's lymphoma. CT scan showed extensive bibasilar reticular opacities with honey combing suggestive of pulmonary fibrosis with Usual interstitial pneumonia pattern. Based on temporal relations to bleomycin chemotherapy, a diagnosis of bleomycin induced lung fibrosis was made. Bleomycin was subsequently discontinued with some improvement of symptoms.
Conclusion: Pulmonary toxicity with irreversible lung fibrosis is a life threatening and menacing adverse effects in patients receiving Bleomycin therapy. The probability of developing lung toxicity from Bleomycin is increased in older patients receiving multiple cycles of chemotherapy, with risk potentially outweighing benefits in select patients with early-stage lymphoma and favorable prognosis.
Bleomycin is an anti-tumor antibiotic indicated in the treatment of lymphomas, germ cell tumors and malignant pleural effusion [1]. It is produced by Streptomyces verticillus and was first isolated by Umezawa, et al. in 1966 [2]. Its mechanism of action involves breakdown of DNA double helix by the production of free radicals, which is dependent on oxygen and iron [2]. Although dermatological adverse effects are the most observed, Bleomycin induced lung toxicity crop up in up to 10% patients which can be life menacing if not diagnosed early with mortality rate of up to 20% [1,3].
Bleomycin induced lung injury encompasses several clinical syndromes including hypersensitivity, bronchiolitis obliterans with organizing pneumonia, hypersensitivity pneumonitis which may progress to pulmonary fibrosis [3]. Several factors including older age and cumulative drug dose have been shown to increase the risk of developing pulmonary toxicity from Bleomycin [4]. The following is a case of pulmonary fibrosis following chemotherapy with Bleomycin for Stage 2B Hodgkin's Lymphoma (HL). This case emphasizes the need for careful selection of chemotherapy regimen in older patients with Hodgkin's lymphoma who are risk for bleomycin pulmonary toxicity and the necessity for diligent surveillance in patients with increased baseline risks for toxicity.
A 64-year-old male from Bangladesh presented to the emergency department with gradual onset of exertional shortness of breath of 1 week which has progressively worsened to shortness of breath at rest. He had a history of dry cough of 1 month but denied fever, chills, pleuritic chest pain, hemoptysis, orthopnea, paroxysmal nocturnal dyspnea or leg swelling. He was diagnosed with HL Stage 2B nodular sclerosis subtype 5 months earlier after presenting to the hematology and oncology department with weight loss, low grade fever and neck mass. At that time, CT neck and chest revealed large necrotic mass on the right side of the neck with multiple enhancing lymph node in the neck, adjacent to the trachea and carina and paraesophageal region; ESR was elevated at 120 mm/hr and diagnosis was confirmed with biopsy of neck mass. At that time, he was started on ABVD regimen for treatment which consists of Adriamycin (Doxorubicin) 42.25 mg (25 mg/m2), Bleomycin 17 units (10 units/m2), Vinblastine 10 mg (6 mg/m2) and Dacarbazine 634 mg (375 mg/m2) all on Day 1 and day 15 per cycle every 4 weeks to complete 6 cycles of treatment, with pegfilgrastim on Day 2 and Day 16 per cycle. Patient had completed 4 cycles of chemotherapy prior to onset of exertional dyspnea. He has no significant medical history prior to being diagnosed with Hodgkin's lymphoma and denies history of cigarette smoking.
He had a baseline pulmonary function test before commencement of chemotherapy which showed normal lung volumes, FEV1 and FVC were 82% and 80% of predicted values respectively and mild reduction in diffusing capacity for carbon monoxide. PET scan done at onset of exertional dyspnea and one-week prior to presentation to the emergency department showed hypermetabolic activity in the lung periphery without residual mass or metabolic uptake in the neck and mediastinum (Figure 1). Initial evaluation, revealed blood pressure of 127/82 mmHg, heart rate of 104 beats per minute, respiratory rate of 22 breaths per minute, SpO2 90% on room air and Temperature was 97.8 F. He had inspiratory rales bilaterally and use of accessory muscles of respiration on examination. Pertinent negatives on examination were absence of ascites, leg swelling, distended neck veins, neck mass, rales, wheezes or stridor. Complete blood count, Basic metabolic panel, procalcitonin, Pro BNP and troponin T values were unremarkable. Chest x-ray shows bilateral interstitial disease prominent at the lung bases. Computed tomography angiography of the neck and chest showed moderate subpleural thickening bilaterally with some ground glass opacity and extensive honeycombing at the lung bases suggestive of usual interstitial pattern (UIP) (Figure 2). No pulmonary embolus, mass, mediastinal, hilar or axillary adenopathy were present on CT scan. Echocardiogram was unremarkable with normal Right and left ventricular function. Sputum culture and markers of connective tissue diseases and vasculitidies were negative. A diagnosis of Bleomycin induced pneumonitis and lung fibrosis was made based on CT findings and temporal relationship with Bleomycin chemotherapy and negative infectious work up. Patient was admitted to the medical floor and started on Prednisolone at 1 mg/kg per day for 4 weeks and subsequent taper. He also received Trimethoprim-sulfamethoxazole for Pneumocystis Jirovei prophylaxis while on steroids. Shortness of breath improved with steroid treatment. However, he desaturates to low 80s on SpO2 during 6 minutes' walk test. He was discharged home one week later with supplemental oxygen during exercise. Based on negative PET and CT findings for active Hodgkin's lymphoma, patient was chemotherapy was discontinued. In addition, patient did not receive consolidative radiation therapy due to extensive lung fibrosis. Unfortunately, patient continued to experience limitation of exercise due to exertional dyspnea with modified medical research Council Scale grade 3 at 5 months follow up. Repeat Pulmonary function test at that time showed restrictive lung pattern with FEV1 and FVC 40% and 34% of predicted values respectively (Table 1).
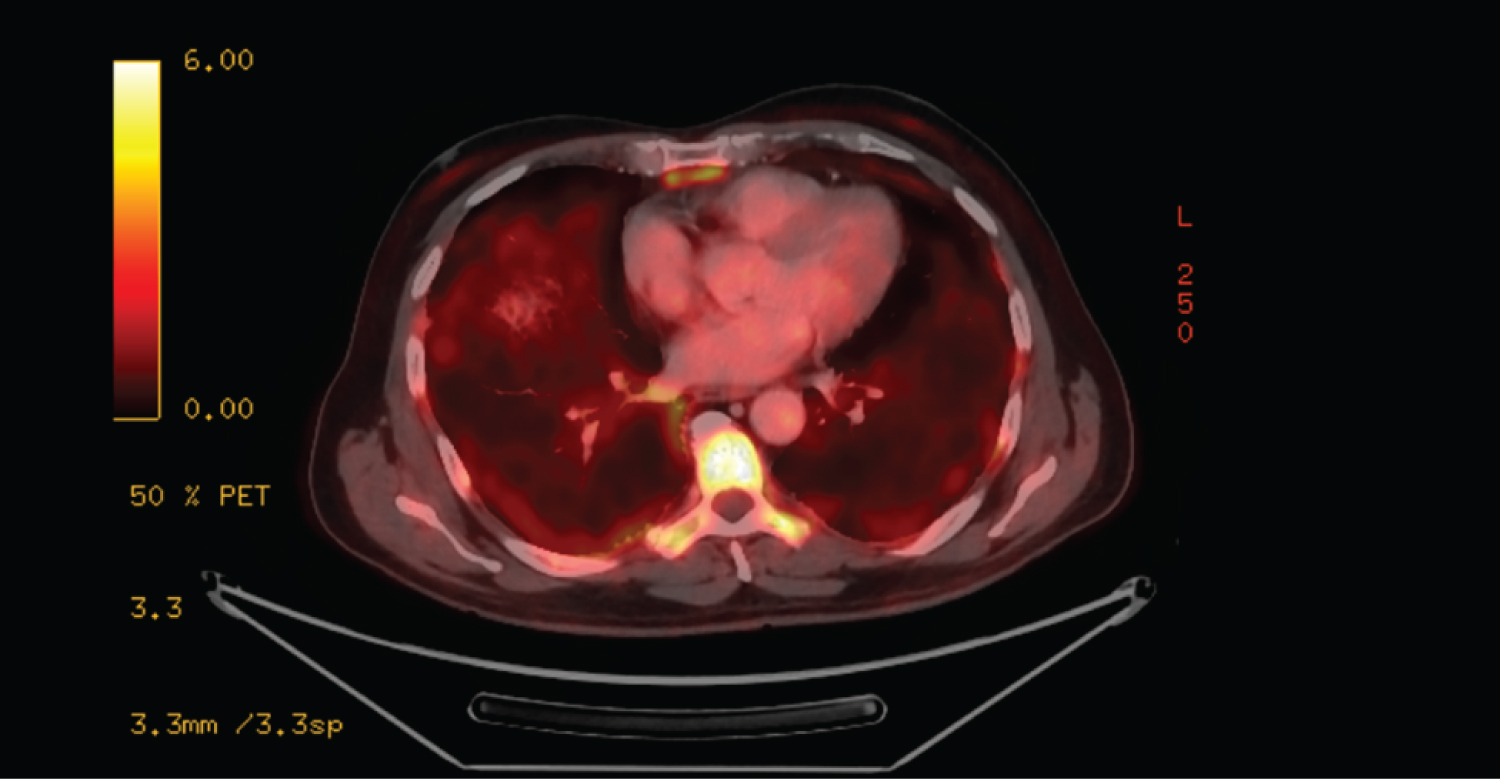 Figure 1: PET scan showing increases hypermetabolic activity in the periphery of the lungs.
View Figure 1
Figure 1: PET scan showing increases hypermetabolic activity in the periphery of the lungs.
View Figure 1
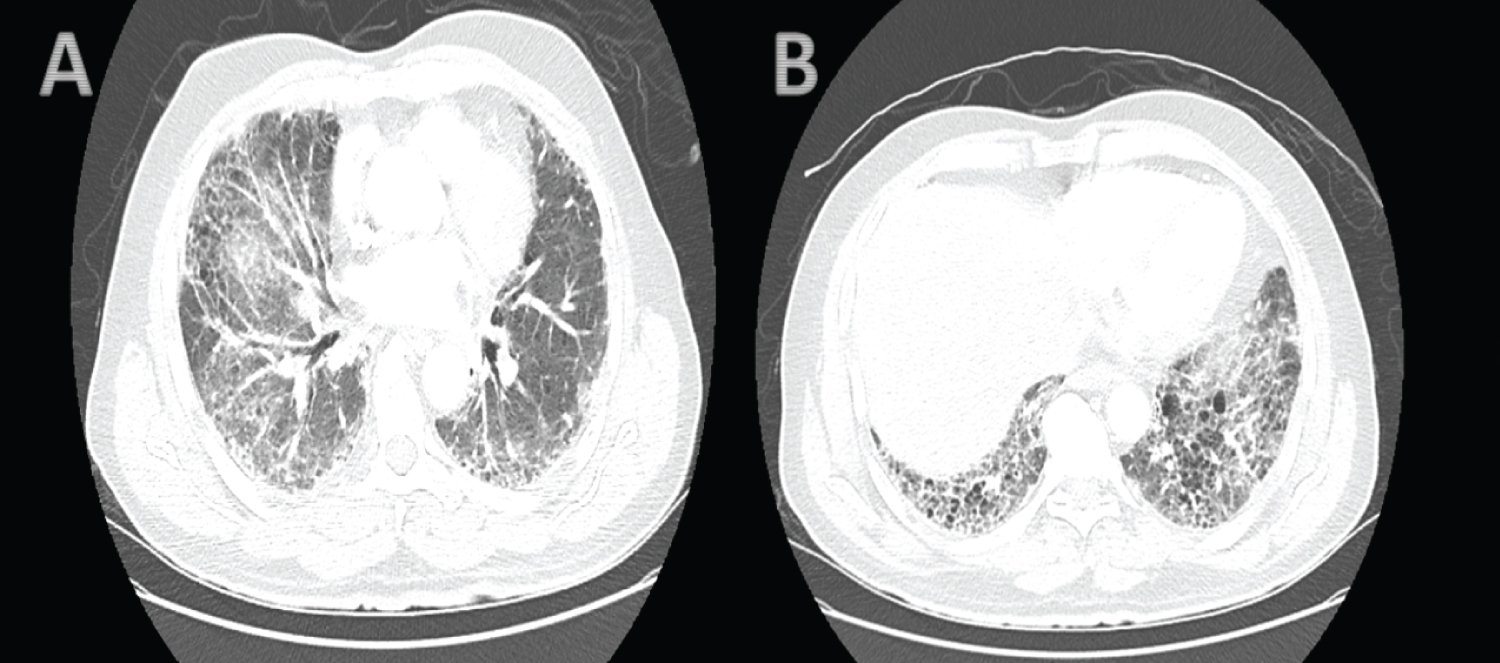 Figure 2: Computed tomography scan of the chest showing moderate subpleural thickening bilaterally with some ground glass opacity (A) and extensive honeycombing at the lung bases (B).
View Figure 2
Figure 2: Computed tomography scan of the chest showing moderate subpleural thickening bilaterally with some ground glass opacity (A) and extensive honeycombing at the lung bases (B).
View Figure 2
Table 1: Pulmonary function test pre and post chemotherapy. View Table 1
Lung injury ranging from pneumonitis to extensive fibrosis is a potential complication of bleomycin therapy, but the extent of this adverse effect has not been extensively documented. Bleomycin-induced pneumonitis (BIP) occurs in 0 to 46% of patients with long-term functional respiratory impairment reported in approximately 15 to 18% of cases [3]. Although substantial recovery is expected in patients with subacute or acute BIP with appropriate management, some patients develop more indolent pulmonary fibrosis. The true incidence of Bleomycin induced lung fibrosis is unknown as this complication can occur 2 to 10 years after discontinuing Bleomycin [5,6]. The pathophysiology of Bleomycin induced lung toxicity is not entirely clear, but it is thought to involve oxidative damage and relative deficiency of deactivating enzyme bleomycin hydrolase in the lungs [2,5,7,8]. Bleomycin is an integral part of combination chemotherapy used in the management of Hodgkin lymphoma (HL), an uncommon B-cell malignant neoplasm accounting for approximately 10.2% of all lymphomas in the United States. Nodular sclerosis Hodgkin lymphoma (NSHL) is the most common subtype of classical HL, accounting for 65% to 75% of cases [9]. Unfortunately, there is still conflicting data regarding how best to prevent complications extending from potentially fatal pneumonitis to irreversible and debilitating complication of pulmonary fibrosis in patients receiving Bleomycin containing chemotherapeutic regimen without compromising treatment efficacy. This is in part due to varying trial designs and treatment endpoints.
Advances in chemotherapeutic agents and radiotherapy have improved patient outcomes; however, careful staging, risk stratification and consideration for potential treatment related adverse events are required in determining optimal standard of care. Prognostication and selection of treatment modalities is based on classification of Hodgkin's lymphoma into early-stage favorable, early-stage unfavorable, and advanced stage. Early-stage Hodgkin lymphoma refers to Ann Arbor stage I or stage II disease. According to study groups (the European Organization for the Research and Treatment of Cancer (EORTC) and the German Hodgkin Study Group), early-stage Hodgkin lymphoma is stratified into a high risk (unfavorable) group defined by any of the following: a large mediastinal mass (one third of maximum thoracic diameter), any mass of ≥ 10 cm in the largest dimension, extra-nodal disease, 3 or more nodal areas, and an ESR of > 50 mm/hr in asymptomatic patients or > 30 mm/hr in patients with B symptoms, age greater than 50 years of age, intraabdominal disease [8].
Although CT scans of the neck, chest, abdomen, and pelvis is included as part of initial diagnosis for staging, positron emission tomography (PET) scanning has become crucial in the initial evaluation, staging and appraisal of treatment response in Hodgkin lymphoma [10]. In the last one hundred years, evolving therapeutics has transformed Hodgkin's lymphoma from a fatal disease to one that is curable in approximately 75% of cases but treatment related toxicities are now an important cause of late morbidity and mortality [11]. To achieve high cure rate and minimize therapy related toxicity, patients with early-stage disease are usually managed with less intensive therapy, while more intensive therapy is reserved for patients with advanced stage disease. However, the definition for intensive and less intensive therapy and criteria for patient selection into therapy group still varies [11,12]. As described above, patients with early-stage HL with favorable prognosis are often managed with less intense regimen options including ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) for three to four cycles, followed by involved-site radiation therapy; ABVD for two cycles, followed by involved-site radiation therapy; ABVD for four to six cycles without radiation for patients at risk of long-term complications from radiation therapy. Patients with unfavorable prognosis stage I to II HL are treated with chemotherapy followed by involved-site radiation therapy in a combined modality program. In most studies, combined modality therapy with ABVD and consolidation radiotherapy remains the "gold standard" chemotherapy for these patients [10,13].
Despite promising outcomes in terms of survival and low relapse rate in patients with bleomycin containing regimen, pulmonary toxicity are drawbacks. A systematic literature review of the burden of bleomycin therapy concluded that about 4%-5% of Hodgkin lymphoma patients had fatal pulmonary toxicity [14]. Unfortunately, the optimal therapy for bleomycin-induced lung toxicity has not been documented. It has been observed from review of literature that older patient with Hodgkin's lymphoma developed severe bleomycin lung toxicity especially after 2 cycles of chemotherapy similar to what was observed in our patient [15-17]. In addition to older age and cumulative dose, other factors that increases risk of Bleomycin toxicity hence needing consideration in constituting a treatment regimen includes renal insufficiency, genetic predisposition, underlying lung disease, concomitant radiotherapy and G-CSF4, [15,18]. In attempts to minimize or avoid bleomycin induced pulmonary toxicity, Identification of risk factors, screening asymptomatic patients for toxicity, role on non-bleomycin containing regimes, early removal of bleomycin from treatment regimens and PET guided therapy are options that have been explored.
Removal of bleomycin after negative findings on interim PET or after 2 cycles of chemotherapy has been shown to reduce the risk of bleomycin toxicity and may have no impact on treatment efficacy, overall survival and progression-free survival according to some studies [16,19]. An open label, randomized, multicenter trial (HD13) of 1502 qualified patients randomly assigned to receive 2 cycles of ABVD, ABV, AVD, or AV with primary endpoint freedom from treatment failure after 5 years. The conclusion from that study was that neither Bleomycin nor Dacarbazine can be safely completely omitted without compromising the standard of care for patients with early-stage favorable Hodgkin's lymphoma [20]. A study of patients with unfavorable early stage cHL randomized patients into EVE regimen (epirubicin, vinblastine, and etoposide) and ABVD regimen with hopes of reducing toxicity showed that patients who received EVE had a significantly worse outcome than those who received ABVD in terms of relapse-free survival [21].
Outcomes from these studies suggests that Bleomycin is a crucial component of front-line chemotherapy for cHL and may be safely omitted after 2 cycles in carefully selected patients receiving more than 2 cycles to achieve efficacy, reduce relapse and reduce toxicity. Although, long-term data are still required, other strategies that are being investigated to avoid bleomycin toxicity involves adding anti-CD30 monoclonal antibody brentuximab vedotin to AVD chemotherapy in the front-line treatment of cHL [22]. Current guidelines for management of 18Ffluorodeoxyglucose-avid lymphomas have adopted PET for adaptation of treatment in addition to staging [23,24]. Negative finding on interim (early) PET after 2 cycles of chemotherapy has enabled selection of patients needing less intensive therapy which includes omission of bleomycin from subsequent treatment, shortening chemotherapy and/or avoiding the adjuvant radiotherapy, resulting in lower incidence of pulmonary toxic effects [19,25,26]. Although concomitant use of bleomycin and radiotherapy may increase pulmonary toxicity, studies suggest that removing radiotherapy is associated with increased risk of relapse and focus should be on reducing the cumulative dose of Bleomycin [20,27].
The importance of early diagnosis of bleomycin induced pneumonitis is crucial to prevent irreversible damage and life-threatening lung fibrosis. Lung volumes typically show a restrictive pattern with decreases in forced vital capacity (FVC), total lung capacity (TLC), functional residual capacity (FRC) and DLCO. Omitting bleomycin is usually recommended for patients with decline in DLCO of 25 percent or greater relative to baseline during treatment [4]. Although cheap and readily available, Pulmonary function tests are unreliable for identifying risk of bleomycin toxicity necessitating more sensitive screening method for early detection. In addition to aiding adaptation of therapy, interim PET also has a role in detection of bleomycin induced toxicity. A study of 77 patients with HL identified 13 patients, 6 of whom were asymptomatic with abnormal lung uptake of fluorodeoxyglucose predominantly diffuse, bilateral, in the lower lobes and subpleural areas. One asymptomatic patient died of bleomycin toxicity while remaining 12 showed resolution of the pneumonitis with steroids and discontinuation of bleomycin [28]. Other reports have also demonstrated that early interim FDG-PET may be more sensitive than CT scanning and could be beneficial for alerting clinicians to asymptomatic and early development bleomycin-induced toxicity thereby reducing fatal outcomes and progression to irreversible lung fibrosis [29,30].
Pulmonary toxicity leading to lung fibrosis, typically irreversible is unfortunately a potential risk especially in older patients receiving more than 2 cycles of bleomycin chemotherapy with risk of lung toxicity potentially outweighing benefits in early-stage HL. Reducing Bleomycin cumulative dose is a viable therapeutic option especially with increasing role of early interim PET adapted therapy enabling early discontinuation of Bleomycin. In addition, frequent clinical monitoring for onset of symptoms, changes in pulmonary lung function tests and interval changes on lung imaging including PET should prompt evaluation for bleomycin induced lung toxicity with the aim of early detection. Prompt discontinuation of Bleomycin is essential in all patients with suspected or documented bleomycin induced lung-toxicity to prevent irreversible lung fibrosis.